Bonding of the silane containing multi-mode universal adhesive for lithium disilicate ceramics
Article information
Abstract
Objectives
This study evaluated the influence of a multi-mode universal adhesive (MUA) containing silane (Single Bond Universal, 3M EPSE) on the bonding of resin cement to lithium disilicate.
Materials and Methods
Thirty IPS e.max CAD specimens (Ivoclar Vivadent) were fabricated. The surfaces were treated as follows: Group A, adhesive that did not contain silane (ANS, Porcelain Bonding Resin, Bisco); Group B, silane (S) and ANS; Group C, hydrofluoric acid (HF), S, and ANS; Group D, MUA; Group E, HF and MUA. Dual-cure resin cement (NX3, Kerr) was applied and composite resin cylinders of 0.8 mm in diameter were placed on it before light polymerization. Bonded specimens were stored in water for 24 hours or underwent a 10,000 thermocycling process prior to microshear bond strength testing. The data were analyzed using multivariate analysis of variance (p < 0.05).
Results
Bond strength varied significantly among the groups (p < 0.05), except for Groups A and D. Group C showed the highest initial bond strength (27.1 ± 6.9 MPa), followed by Group E, Group B, Group D, and Group A. Thermocycling significantly reduced bond strength in Groups B, C, and E (p < 0.05). Bond strength in Group C was the highest regardless of the storage conditions (p < 0.05).
Conclusions
Surface treatment of lithium disilicate using HF and silane increased the bond strength of resin cement. However, after thermocycling, the silane in MUA did not help achieve durable bond strength between lithium disilicate and resin cement, even when HF was applied.
Introduction
All-ceramic restorations have gained popularity because of their biocompatibility and translucency as well as good esthetics.12 The chosen material for all-ceramic restorations has shifted from pressed ceramic to monolithic ceramic to improve the mechanical properties. Monolithic ceramic, lithium disilicate, is popular because it provides good esthetics and better chipping fracture resistance relative to non-monolithic materials such as porcelain-veneered zirconia.3 It also has greater strength than other ceramic materials such as leucite glass and metal ceramics.4
The clinical outcomes of ceramic restoration do not depend only on the properties of the material, but also on the resin-ceramic bond. Strong and durable resin bonding increases retention,56 improves marginal adaptation,78 reduces microleakage89 and enhances fracture resistance.10 This resin-ceramic bond is created through micromechanical retention and chemical bonding to a silica-based ceramic surface.1112 To produce micromechanical retention, the surface is prepared by airborne-particle abrasion and/or etching with hydrofluoric acid (HF). However, airborne-particle abrasion is not recommended due to a significant reduction in the flexural strength of IPS e.max CAD (Ivoclar Vivadent, Schaan, Liechtenstein).13 HF etching dissolves the glass phase from the matrix, thus creating micro-undercuts and increasing the surface area.14 Chemical bonding between the resin-ceramic surfaces can be achieved using a silane coupling agent. Silane is a bifunctional molecule that promotes adhesion via covalent bonds with hydroxyl (OH) groups on the ceramic surface.15 One functional group can react with the inorganic ceramic surface and the other is a methacrylate group capable of reacting with an organic resin matrix.16
Recent trend in adhesive dentistry is to simplify bonding procedures by reducing the application steps.17 Accordingly, many manufacturers have introduced new single-bottle adhesives called ‘universal’ or ‘multi-mode universal’ adhesives (MUAs). They contain many ingredients, such as bisphenol A glycidyl methacrylate (Bis-GMA), hydroxyethyl methacrylate (HEMA), 10-methacryloyloxydecyl dihydrogen phosphate (MDP), and/or silane. However, MDP and silane are usually not included in conventional ceramic adhesives. As MDP is a versatile amphiphilic functional monomer, it is the most important component in MUAs for practical use. MDP has the potential to bond chemically to metals,18 zirconia,19 and tooth tissue.20 Furthermore, it possesses the ‘ideal’ bonding agent property, that the polar phosphate group of the functional monomer is initially hydrophilic, but MDP becomes more hydrophobic once polymerized.21 Several studies investigated the bond strength of MUAs applied on several materials such as enamel,22 dentin,23 zirconia,2425 and ceramics.1026 The manufacturer of MUAs containing silane proposes improved bonding to glass ceramics or resin composites without additional priming procedures. However, little is known regarding the bonding efficiency of MUAs to lithium disilicate ceramic with thermocycling compared to when silane and adhesive were used separately.
The purpose of our current study was to investigate the effects of silane containing MUA on the bonding of resin cement to lithium disilicate ceramic using the microshear bond strength (µSBS) test. The null hypotheses tested were: (1) silane containing MUA does not increase the bond strength between lithium disilicate and resin cement compared to when silane and adhesive are used separately and (2) thermocycling does not affect µSBS between lithium silicate ceramic and resin cement.
Materials and Methods
Specimen preparation
Thirty 12 mm × 14 mm × 5 mm IPS e.max CAD blocks were fabricated (Table 1). The blocks were sintered in a furnace (Horizon Press, ShenPaz Dental Ltd., Migdal Haemek, Israel) according to the manufacturer's instructions. After cooling, each specimen was embedded into an acrylic resin block. The lithium disilicate surfaces were sequentially polished with 120, 220, and 500 grit silicon carbide paper using an automatic polishing machine (Rotopol-V, Struers, Ballerup, Denmark) under water cooling. The specimens were treated with 10% citric acid27 and cleaned in an ultrasonic water bath for 10 minutes to remove the smear layer, and then dried under vacuum for 24 hours.
Surface treatment of lithium disilicate blocks
The specimens were randomly divided into five groups (Figure 1). The lithium disilicate surface of each specimen was treated as follows:
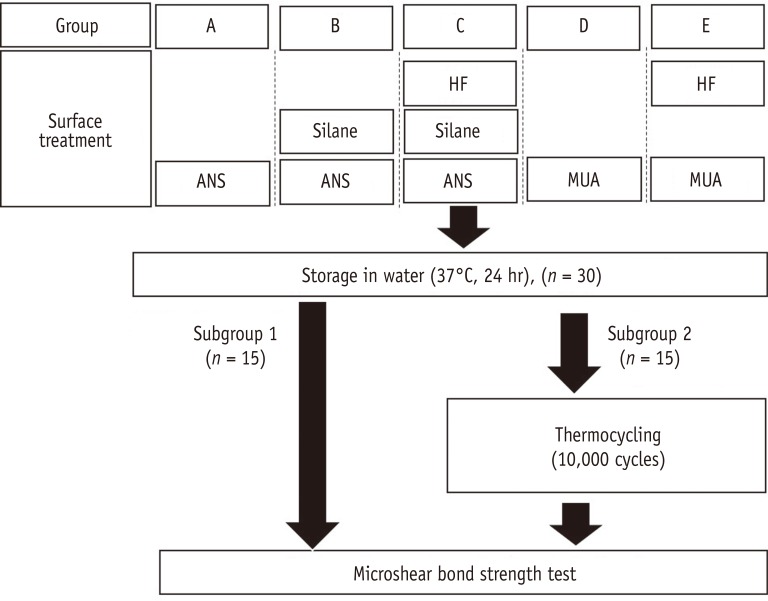
Experimental design of the study. HF, Hydrofluoric acid; ANS, adhesive that does not contain silane (Porcelain Bonding Resin, Bisco); MUA, Multi-mode universal adhesive (Single Bond Universal, 3M EPSE).
Group A (ANS, control): an adhesive that did not contain silane (ANS, Porcelain Bonding Resin, Bisco Inc., Schaumburg, IL, USA) was applied with a microbrush.
Group B (S + ANS): silane (S, Bis-Silane, Bisco Inc.) was applied and air-dried, followed by application of ANS as above.
Group C (HF + S + ANS): 5% HF (Ceramic Etching Gel, Ivoclar Vivadent) was applied for 20 seconds, rinsed with distilled water for 1 minute, and air-dried. Then, S and ANS were applied in the same manner as in Group B.
Group D (MUA): The surface was treated with MUA (Single Bond Universal, 3M EPSE, St. Paul, MN, USA) and agitated via scrubbing with a microbrush for 20 seconds. It was then gently air-dried for 5 seconds.
Group E (HF + MUA): 5% HF was applied for 20 seconds, rinsed with distilled water for 1 minute, and air-dried. Then, the surface was treated with MUA in the same manner as in Group D.
Cementation of the composite to the ceramic
Dual-cure resin cement that did not contain amine (NX3, shade clear, Kerr Corp., Orange, CA, USA) was applied and pre-cured composite resin cylinders (Filtek Z250, 3M EPSE) with diameters of 0.8 mm were placed on six treated ceramic surfaces in each group under a fixed load of 0.4 N. After excess cement was removed with microbrushes, the resin cement was light-cured for 40 seconds with an LED light-curing machine at wavelengths of 430 - 490 nm (Be Lite, B&L Biotech, Ansan, Korea).
Storage of the specimens
All specimens were stored in distilled water at 37℃ for 24 hours and were divided randomly into two subgroups. Half were subjected to µSBS testing (n = 15), and the remainder were underwent for 10,000 thermal cycles with a dwell time of 24 seconds and transfer time of 6 seconds between 5 and 55℃ water baths and subjected to µSBS testing (n = 15).
Microshear bond strength testing
The specimens were mounted in the jig of a universal testing machine (UTM, LF Plus, Lloyd Instruments Ltd., Fareham Hampshire, UK). A wire of 0.2 mm in diameter was looped around the resin composite cylinder as closely as possible to the bonded interface. The shear force was applied at a cross-head speed of 0.5 mm/min until failure occurred. If premature failure occurred before bond strength testing, the bond strength was recorded as 0 MPa.
Failure analysis
After µSBS testing, the fractured interfaces of the specimens were observed using a stereomicroscope (SZ-PT, Olympus Co., Tokyo, Japan) at ×40 magnification to determine the failure mode. The failure mode was classified as ‘adhesive failure’ when it occurred between the ceramic and the resin cement, ‘cohesive failure’ when it occurred within the ceramic or resin, and ‘mixed failure’ when a combination of adhesive and cohesive failures occurred. The representative fractured specimens were examined in a field-emission scanning electron microscope (FE-SEM, S-4700, Hitachi High technologies Co., Tokyo, Japan) operated at 15 kV.
Microscopic observation of bonded interfaces
To observe the bonded interfaces among the lithium disilicate ceramic, adhesive, and resin cement, 3 mm × 5 mm × 14 mm IPS e.max stick specimens were prepared. The ceramic surfaces were treated according to the procedures for each group. The resin cement was applied and light-cured for 40 seconds. All specimens were stored in water for 24 hours, and half were subjected to 10,000 cycles of thermocycling. To observe the bonding quality, the middle point of each stick specimen was fractured in compression mode with a UTM. The fractured surfaces of the sticks were observed by the same FE-SEM.
Statistical analysis
Bond strength data were statistically analyzed using multivariate analysis of variance with statistical software (SPSS version 18.0, SPSS Inc., Chicago, IL, USA). Multiple comparisons were performed using the Tukey's post hoc test, where a p value less than 0.05 was considered statistically significant.
Results
The mean bond strength values and standard deviations are presented in Table 2. Before thermocycling, the bond strength of Group A (1.35 ± 1.12 MPa) was the lowest. The bond strength of Group D (1.53 ± 0.61 MPa) was similar to that of Group A. Groups B (8.66 ± 2.83 MPa), C (27.14 ± 6.85 MPa), and E (21.37 ± 5.08 MPa), which were treated with either HF or silane, showed higher bond strength of the resin cement to the ceramic surface than Group A (p < 0.05).
During the thermocycling procedure, all specimens of Groups A and D were spontaneously debonded. After thermocycling, the bond strengths decreased in Groups B, C, and E (p < 0.05). Group C showed the highest bond strengths regardless of the storage conditions (p < 0.05).
The distribution of failure modes after µSBS testing is presented in Figure 2. The mode of failure was all adhesive failure in Groups A and D. However, cohesive failures occurred in the HF-treated groups (33.3 and 26.7% in Groups C and E, respectively). After thermocycling, 100% of the failures were adhesive in Groups B and E, whereas mixed failures (33.3%) occurred in Group C. The mean shear bond strength for Group C was significantly higher than that of Group E (p < 0.05), and Group C had fewer adhesive failures than Group E did. Figure 3 shows representative SEM images of e.max surface after µSBS tests.
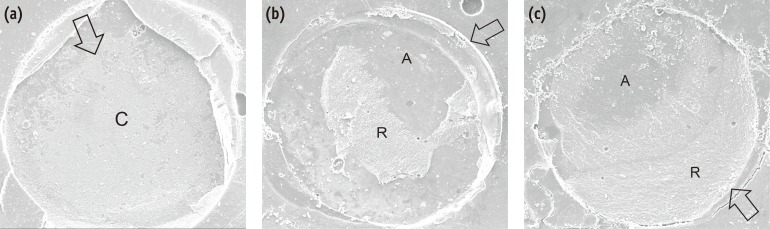
Representative SEM photomicrographs of fractured ceramic surfaces after microshear bond strength testing showing (a) adhesive failure; (b) mixed failure; (c) cohesive failure at ×100 magnification. The arrow shows the fracture origin and the direction of the arrow represents that of shear force. In Figure (c), the resin cement remained on the loading point side. C, ceramic; A, adhesive; R, resin cement.
While the specimens were being processed for microscopic observation of the bonded interfaces, all specimens spontaneously debonded in Groups A and D before thermocycling, and in Groups B and E during thermocycling. There was no gap between the ceramic and adhesive in Group C before or after thermocycling (Figures 4b and 4d) compared to Groups B and E before thermocycling (Figures 4a and 4c).
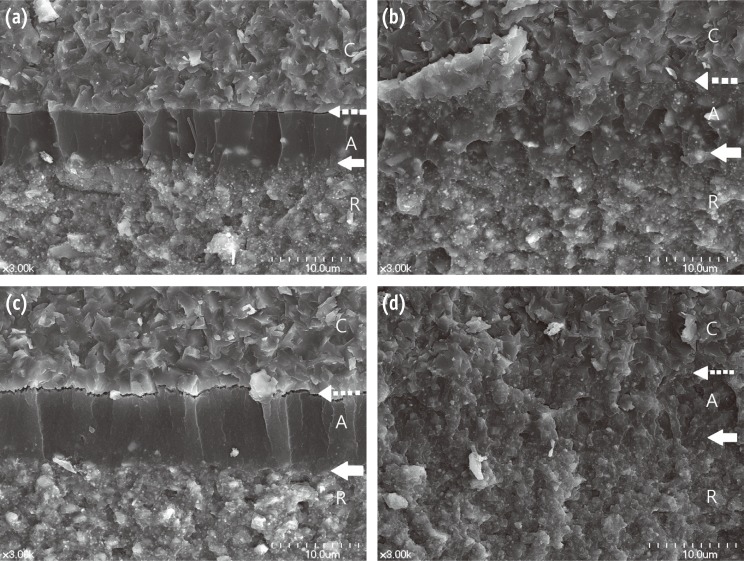
SEM micrographs of the fractured surfaces comparing the adaptation between the adhesive and the ceramic surfaces treated with different procedures: (a) Group B (silane, adhesive that did not contain silane [ANS], and resin cement) before thermocycling. The surface of the lithium disilicate ceramic was flat, and there was no micro-undercut, because hydrofluoric acid (HF) had not been applied. The adhesive and resin cement layers can be discriminated. There were some filler particles in the adhesive layer; (b) Group C (HF, silane, ANS, and resin cement) before thermocycling. The borders of each material were not easily distinguishable because the adhesive had infiltrated the micro-undercut and the fillers were distributed throughout the full thickness of the adhesive; (c) Group E (HF, multi-mode universal adhesive [MUA], and resin cement) before thermocycling. The etched ceramic surface had micro-undercuts and MUA had infiltrated the undercuts. However, there was a gap between the adhesive and the ceramic surface; (d) Group C (HF, silane, ANS, and resin cement) after thermocycling. This had a similar morphology to Figure 4b. Dashed arrow, the interface of the ceramic and adhesive; hollow arrow, the interface of the adhesive and resin cement. C, ceramic; A, adhesive; R, resin cement.
Discussion
We sought to examine the efficacy of MUA on the bond strength of resin cement to lithium disilicate ceramic using µSBS testing. Several testing methods are used to evaluate the bond strength between different materials, such as shear bond strength (SBS), tensile bond strength (TBS), µSBS, and microtensile bond strength (µTBS) tests. The larger the bonding area is, the higher the likelihood of a flaw being present and the lower the bond strength.28 Either µSBS or µTBS tests are the most common approach. The µTBS test requires a uniform stress distribution during loading.29 However, it is difficult to fabricate microbeam specimens with sintered IPS e.max CAD blocks without damaging the bonded interface. Conversely, µSBS specimens are pre-stressed prior to testing only by mold removal.30 Therefore, in this study, the µSBS test method was used, because it is not only a simple and reproducible procedure,31 but it also permits efficient screening of adhesive systems.28
Single Bond Universal has a low pH of 2.7 due to a MDP. When it is mixed with self-cured resin cement, an acid-base reaction occurs between MDP and an aromatic tertiary amine which is the activator of chemical polymerization. The consequence of this reaction is lack of polymerization at the adhesive-cement interface. Therefore, the manufacturer recommends mixing it with a separate activator if another manufacturer's self- or dual-cure resin cement is used,21 or to use it with amine-free dual-cured resin cement. When Single Bond Universal is used with its activator, it is obtained in two different bottles. This does not have any advantages compared to a separate silane and adhesive system, like Group C. To simplify the process, we chose to use an amine-free dual-cured resin cement, NX3.
The application of MUA alone showed similar bond strength compared to Group A. All specimens in Group D were debonded during thermocycling (Table 2). The first hypothesis was accepted. One possible explanation for this interesting finding is impairment of silane stability in the acidic environment.32 Because the pH of MUAs is 2.2 to 3.2 for self-etching capability, a self-condensation reaction occurs in the silanol groups of hydrolyzed silane.3334 A second possible explanation is that Bis-GMA in MUAs significantly inhibits the condensation reaction between the hydroxyl groups of lithium disilicate ceramic and the silanol groups of silane.35 Moreover, extra resin could inhibit the condensation reaction that releases water molecules according to the Le Chatelier principle.36 Furthermore, Uncured HEMA lowers the vapor pressure of water and make it difficult to remove water by air-drying.37 Another explanation is that the concentration of silane in MUA might not be sufficient to react with the hydroxyl groups of the ceramic surface. This was confirmed by the studies by Zaghloul et al.35 and Kalavacharla et al.26 According to these authors, treatment with silane followed by MUA significantly improved the bond strength between the ceramic and the composite resin. The additional silanization step enhanced chemical bonding to the exposed hydroxyl groups and surface wettability with resin impregnation.
The results of the current study supported the importance of HF etching prior to ceramic surface bonding. Lower bond strength was obtained if the cement was applied without HF etching of the ceramic surface (Table 2), which confirmed the findings reported in earlier studies.1338 The large difference in bond strength contingent on HF etching is explained by the difference in surface texture (Figures 4a and 4c). HF etching of a ceramic surface dissolves the glass phase and forms soluble hexafluorosilicates, which can be rinsed out with water. In addition, HF etching creates surface irregularities, thus increasing surface area.39 It also exposes OH groups, consequently improving the wettability of the ceramic by silane agents.
In the present study, the application of HF etching, silane, and adhesive showed the highest bond strength in comparison to other experimental groups. This procedure achieved durable bonds for silica-based ceramics.1140 Conversely, Isolan et al.41 reported that the µSBS achieved with MUA was higher than that obtained with HF, silane, and Single Bond 2 (SB2, 3M ESPE) treatment. Although we used Porcelain Bonding Resin, which is HEMA-free, Isolan et al.41 used SB2, which is not. According to El Zohairy et al.,42 bonding agents containing hydrophilic monomers have a negative effect on resin-ceramic bonds. Therefore, SB2 might influence the bond strength during water storage. In addition, different experimental settings, such as the use of different ceramic blocks, could have influenced these results.
Our results showed that durable resin-ceramic bond cannot be obtained by silane application without HF acid etching of the ceramic surface (Table 2). The bond strength in Group B, which was treated with silane and adhesive, was slightly higher compared to that in Group A. This indicated that silane contributes to the resin-ceramic bond, but showed that silane alone is not sufficient to produce durable ceramic bonding. This was corroborated by failure mode data, that is, adhesive failure was more common in Group B than in Group C.
Dental restorations are exposed to a harsh environment, such as repeated occlusal force, moisture and the thermal variation in the oral cavity. These can cause the failure of restorations. In the present study, thermal cycling was used to simulate clinical conditions. The results indicated that we could reject the second hypothesis. The post-thermocycling bond strength decreased significantly in Groups B, C, and E (Table 2). Several studies have proposed that thermocycling might have a negative effect on the bond strength between resins and ceramics.434445 As mentioned above, an adhesive layer including HEMA could influence the resin-ceramic bond durability.42 MUA contains hydrophilic monomers, such as HEMA, that vary from the monomers present in ANS. The deterioration of the bond observed when MUA was used was correlated with the hydrophilic characteristics of the adhesive. Previous studies have proposed that water uptake will diminish the siloxane bond by hydrolysis and water swelling. HEMA has a low partition coefficient (p = 0.26). The more HEMA resin present, the more water is absorbed.4647 The effect of swelling will stress the bond at the adhesive interface and will significantly weaken adhesive bonds.42
In current study, there were gaps in the resin-ceramic interfaces in Group E before thermocycling (Figure 4c), and spontaneous failure occurred in Group E during thermocycling for microscopic observation. Considering the composition of MUA and the gaps, the bond strength of Group E was significantly decreased after thermocycling. In contrast, the ceramic-adhesive-cement interface could not be detected and intimate bonding was observed in Group C before and after thermocycling (Figures 4b and 4d). This was consistent with the results of bond strength testing in Group C.
The main limitation of this study is that only one brand of MUA was tested. There are many MUAs that have different compositions, as well as different ingredients (Table 3). For example, Single Bond Universal and Clearfil Universal Bond (Kuraray Noritake Dental, Tokyo, Japan) include silane, while other MUAs did not incorporate silane. Some adhesives include special ingredients, such as 3 - 7% methacrylated carboxylic acid polymer in Adhese Universal (Ivoclar Vivadent), or 1 - 5% polyacrylic acid copolymer in Single Bond Universal. Each MUA may have different bonding interactions according to the surface treatments used and their ingredients. The resin cement used was NX3, which is not manufactured by the supplier of the MUA. The manufacturer of the MUA recommends using it in combination with Rely X Ultimate cement. Further studies are needed to compare the bond strength of MUA between these two cements.
Conclusions
Micro-undercuts formed by HF etching on lithium disilicate played an important role in the bond strength between lithium disilicate ceramic and resin cement. Silane also contributed to the formation of a durable bond to lithium disilicate. Silane contained in the tested MUA (Single Bond Universal) did not seem to enhance the bonding strength between lithium disilicate ceramic and resin cement.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.






