Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry
Article information
Abstract
Objectives
The purpose of this study was to assess the ability of two new calcium silicate-based pulp-capping materials (Biodentine and BioAggregate) to induce healing in a rat pulp injury model and to compare them with mineral trioxide aggregate (MTA).
Materials and Methods
Eighteen rats were anesthetized, cavities were prepared and the pulp was capped with either of ProRoot MTA, Biodentine, or BioAggregate. The specimens were scanned using a high-resolution micro-computed tomography (micro-CT) system and were prepared and evaluated histologically and immunohistochemically using dentin sialoprotein (DSP).
Results
On micro-CT analysis, the ProRoot MTA and Biodentine groups showed significantly thicker hard tissue formation (p < 0.05). On H&E staining, ProRoot MTA showed complete dentin bridge formation with normal pulpal histology. In the Biodentine and BioAggregate groups, a thick, homogeneous hard tissue barrier was observed. The ProRoot MTA specimens showed strong immunopositive reaction for DSP.
Conclusions
Our results suggest that calcium silicate-based pulp-capping materials induce favorable effects on reparative processes during vital pulp therapy and that both Biodentine and BioAggregate could be considered as alternatives to ProRoot MTA.
Introduction
To preserve vitality of the pulp tissue and to prevent its pathological changes, cariously or mechanically exposed vital pulp must be sealed with a biocompatible material to protect the pulp from additional injury and to promote healing and repair. The healing process of dental pulp is characterized by the formation of a hard tissue and maintenance of vitality without inflammation.12 Calcium hydroxide has played an important role in vital pulp therapy by inducing formation of a hard tissue barrier. However, despite the long history, its use in vital pulp therapy remains controversial because of its destructive cytotoxicity and tunneling of the dentin bridge.3
Mineral trioxide aggregate (MTA) has received attention as an alternative to calcium hydroxide. MTA contains tricalcium silicate, tricalcium aluminate, tricalcium oxide, and silicate oxide. Its ability to stimulate formation of a dentin bridge, consequently leading to pulp healing has been well demonstrated in previous studies.4567 MTA has excellent biocompatibility without mutagenic potential, sealing capacity and the deposition of cementum, which may promote the regeneration of the periodontal tissue and the formation of mineralized tissue.891011121314 However, it has a long setting time and a tendency to become discolored.15
Recently, a new calcium silicate-based cement, Biodentine (Septodont, Saint-Maur-des-Fosses, France), has been introduced. The Biodentine powder contains mainly tricalcium silicate, dicalcium silicate, and calcium oxide, and the liquid consists of calcium chloride and a carboxylate-based hydrosoluble polymer (water-reducing agent). Its prominent characteristics are shorter setting time (12 minutes) and better compressive strength and sealing ability than MTA.16 Biodentine can be applied as a dentin substitute. Previous studies on its interactions with pulp cells demonstrated its biocompatibility and its ability to induce odontogenic differentiation and mineralization in cultured pulp cells.16171819202122 However, its ability to stimulate reparative dentin in direct pulp-capping remains to be studied further.
BioAggregate (Innovative BioCeramix Inc., Vancouver, BC, Canada) is a new bioceramic material for perforation repair and retrograde filling. It mainly consists of tricalcium silicate, dicalcium silicate, calcium phosphate monobasic, amorphous silicone dioxide, and tantalum oxide as a radiopacifier. It is claimed to stimulate cementogenesis, to form a hermetic seal, and to have effects on osteoblast differentiation and odontoblastic differentiation.16212223242526
Although many studies can be found regarding MTA, Biodentine, and BioAggregate, the reactions of pulp tissue to these three materials have not been simultaneously compared histologically. The objective of this study was to evaluate and characterize the reparative dentin formation of MTA, Biodentine, and BioAggregate using micro-computed tomography (micro-CT) and histology, and to provide guidelines for selecting the most appropriate biomaterials for pulp-capping procedures.
Materials and Methods
Surgical procedures
Eighteen Sprague-Dawley rats, 9 weeks old, were evenly divided into three treatment groups (n = 6). The rats were anesthetized with an intraperitoneal injection of 50 mg/kg of Zoletil 50 (Virbac, Carros, France) and 15 mg/kg of Rompun (Bayer, Leuverkeusen, Germany). Class I cavities were prepared to induce pulp exposure on the occlusal surfaces of the left and right maxillary first molars using a 1/4 round bur (n = 36). Bleeding was controlled by applying light pressure with wet cotton pellets and by irrigation with sodium hypochlorite (NaOCl) and sterile saline.
The cavities were then filled with either of MTA (ProRoot MTA, Dentsply Tulsa Dental, Tulsa, OK, USA), Biodentine (Septodont), or BioAggregate (Innovative BioCeramix Inc.), each of which was mixed according to the manufacturers' instructions. The exposed site was acid-etched (ETCH-37, Bisco Inc., Schaumburg, IL, USA), dentin adhesive (Xeno V, Dentsply DeTrey GmbH, Constanz, Germany) was applied, and the area was restored with flowable resin (G-aenial Universal Flo, GC Corp., Tokyo, Japan). After 4 weeks, the animals were anesthetized and sacrificed by means of intracardiac perfusion with 4% paraformaldehyde buffered with sodium cacodylate 0.1 M at pH 7.2 - 7.4. All procedures were performed in accordance with the animal experimental guidelines of the Institutional Animal Care and Use Committee of Chonnam National University Dental Hospital in Korea.
Micro-CT imaging
Block sections including molar specimens were dissected from the maxillas of all the rats and were placed in 10% formalin for storage before micro-CT scanning. Fixed block sections were scanned using a high-resolution micro-CT system (SkyScan 1172, SkyScan, Aartselaar, Belgium). Each section was mounted in a plastic container on the scanning platform with the root oriented vertically. The x-ray transmission was set at 180 degrees of rotation, with the x-ray source set at 70 kV/141 µm. A 0.5 mm aluminum filter was used to cut off the softest x-ray. The raw data were reconstructed into images using SkyScan's cluster reconstruction software (NRecon/NRecon Server).2425 After reconstruction, gray images were displaced by color images to visualize mineral density. The Image J program (version 1.47, National Institutes of Health, Bethesda, MD, USA) was used for quantitative analysis. Areas of newly formed reparative dentin and pulp cavity were measured in five randomly selected transverse sections, and the relative ratio of reparative dentin to pulp cavity was calculated.
Histological examination
Specimens were immersed in a 4% paraformaldehyde solution for 24 hours at 4℃. Samples for demineralization were placed in a decalcifying agent (Calci-Clear Rapid, National Diagnostics, Atlanta, GA, USA) at 4℃ for a month. The tissues were treated with ethanol dehydration, embedded in paraffin, and cut into 5 µm thick sections. Tissue samples were stained with hematoxylin and eosin for evaluation of dentine bridge formation and pulpal inflammation. Slides were scanned with a Panoramic MIDI scanner (3DHISTECH, Budapest, Hungary), and digital image analysis was performed with Panoramic Viewer software (3DHISTECH).
Immunohistochemical examination
Immunohistochemical reactions were performed with a streptavidin-biotin system (LSAB System-HRP, Dako, Glostrup, Denmark) according to the manufacturer's protocol. Briefly, specimens were de-waxed in xylene, rehydrated in a graded alcohol series, placed in an endogenous peroxide blocker for 10 minutes, and washed with Tris-buffered saline (TBS). The primary anti-dentin sialoprotein (DSP) antibody (dilution 1:100) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was incubated at 4℃ for 1 hour, followed by the biotin-streptavidin peroxidase complex. The tissues were counterstained with hematoxylin. A positive control was prepared from samples with no pulp treatment. Negative controls consisted of 1% bovine serum albumin substituted for DSP antibody. The immunostained samples were scanned and reviewed, as described under Histological examination.
Statistical analysis
The results were analyzed by one-way analysis of variation (ANOVA), and the Duncan test was used for post hoc analysis. A p value of less than 0.05 was considered statistically significant.
Results
Micro-CT analysis
In normal dental tissue, green color represents dentin and bone, blue color represents enamel, and purple color represents pulp and soft tissue (Figures 1a and 1b). In the MTA sample, green-colored thick homogeneous hard tissue was observed beneath the pulp exposure site. A complete hard tissue bridge with a high-intensity green hue was uniformly seen (Figures 1c and 1d). The mineral density of this newly formed green tissue was similar to that of dentin. The Biodentine specimens showed calcified materials beneath the pulpotomy site, with the calcification tissue exhibiting different degrees of saturation (light green, Figures 1e and 1f). In the BioAggregate group, newly formed thick hard tissue was also observed at the pulpal floor and lateral wall of the pulp chamber. This newly formed tissue, which had a light-green hue, was unevenly distributed (Figures 1g and 1h).
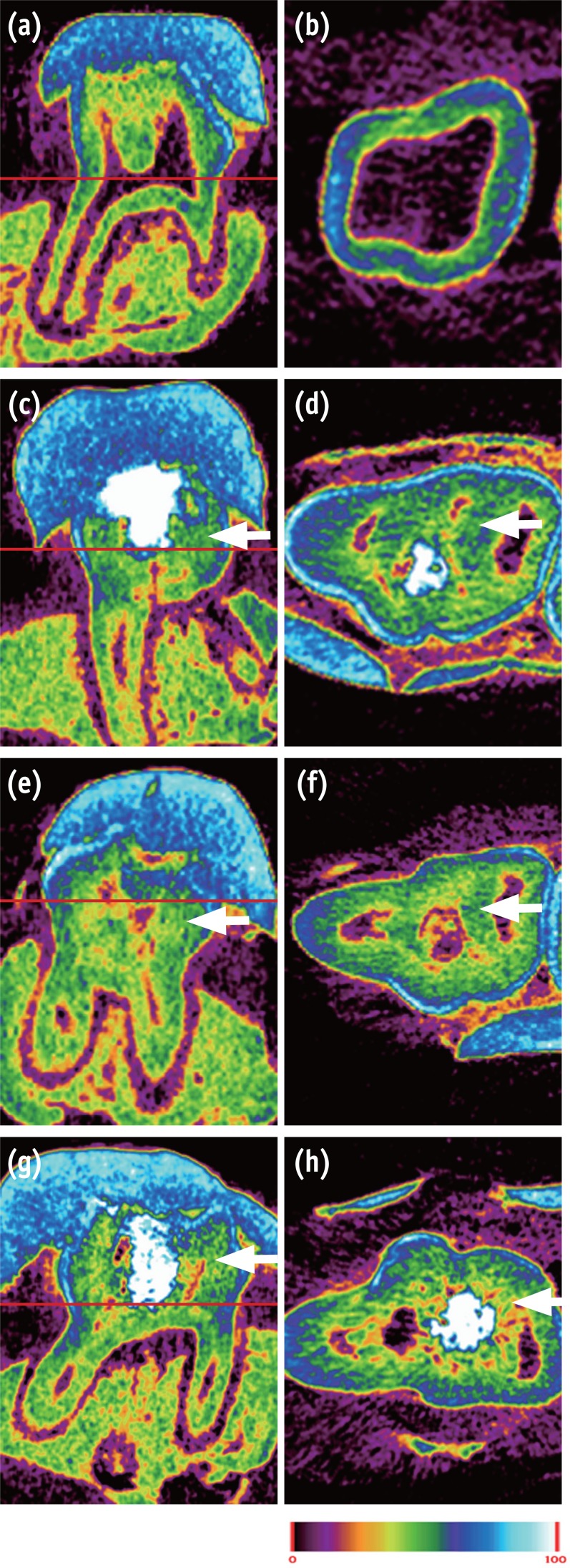
Micro-CT image of pulp capped rat molar teeth after 4 weeks. MTA and Biodentine showed thicker hard tissue formation than did BioAggregate. (a, b) Normal pulp; (c, d) MTA; (e, f) Biodentine; (g, h) BioAggregate group. Color scale bar indicates mineral density from 0 (corresponding to black in radiograph) to 100 (corresponding to white in radiograph). White arrow indicates reparative dentin.
The relative ratios of newly formed reparative dentin to pulp cavity were 0.50 ± 0.02, 0.47 ± 0.03, and 0.44 ± 0.02 for MTA, Biodentine, and BioAggregate, respectively. The difference between MTA and BioAggregate in forming hard tissue was statistically significant (p < 0.05, Figure 2).
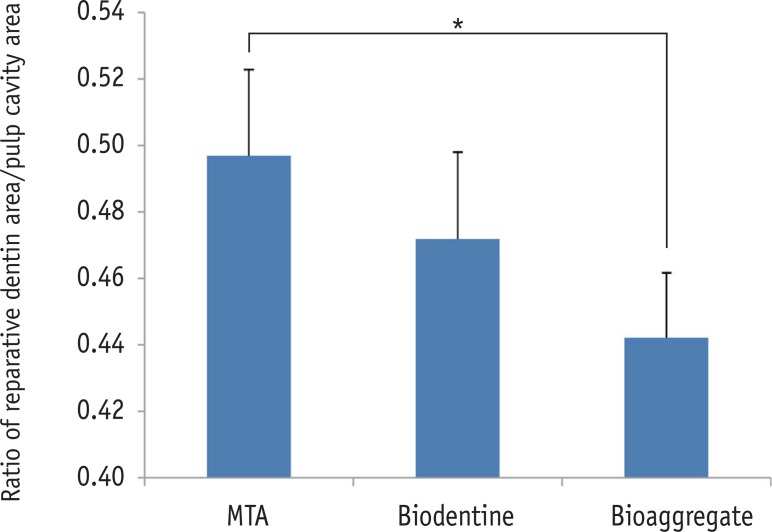
The relative ratio of newly formed reparative dentin to pulp cavity. Area of mineralized tissue and pulp cavity was measured by Image J (version 1.47, National Institutes of Health, Bethesda, MD, USA). MTA and BioAggregate differed significantly in forming a hard tissue.
*p < 0.05.
MTA, Mineral trioxide aggregate.
Histological findings
The results of histological analysis showed that the dentin bridge was created in all three experimental groups. Specimens in the MTA group showed complete calcified bridges. Remaining pulp tissue was vital with an intact odontoblastic layer. Few inflammatory cells were observed beneath the site of pulp exposure in any specimens (Figures 3a and 3b). In all the Biodentine specimens, the pulp wound healed with thick, hard tissue formation. The access cavity was completely closed by the dentin bridge. The hard tissue had an irregular pattern beneath the site of pulp exposure. Necrotic changes in the pulp were not observed (Figures 3c and 3d). The BioAggregate specimens showed a dense calcified area at the pulp exposure interface. Vital pulp was observed without acute inflammation or necrosis (Figures 3e and 3f).
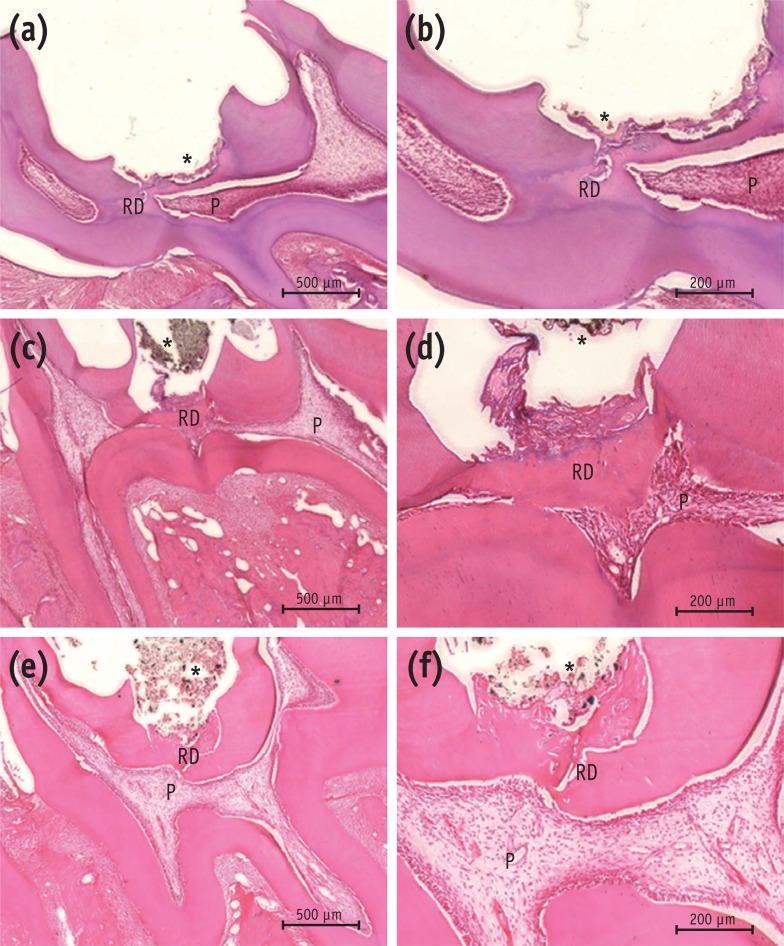
Histological analysis of rat molar teeth. (a, b) MTA; (c, d) Biodentine; (e, f) BioAggregate. At 4 weeks, hematoxylin and eosin stained sections showed reparative dentin bridge formation in all samples. A thick, homogeneous reparative dentin bridge and reactionary dentin could be seen in the MTA group (a, b). Reparative tissue was continuous and thick in the Biodentine group (c, d). Bridges in the BioAggregate group had a dense mineralized structure (e, f).
*Biomaterial.
P, pulp; RD, reparative dentin.
Immunohistochemical findings
Newly formed hard tissues from all experimental groups demonstrated positive immunoreactivity for DSP. In the MTA group, newly formed, strong immunopositive layer was observed within and around the pulp tissue that formed at the exposure site. In the Biodentine group, immunolabeled mineralized tissue was also seen within the pulp exposure site. In the BioAggregate group, there were positive expressions of DSP within the newly formed tissue at the site of the defect (Figure 4).
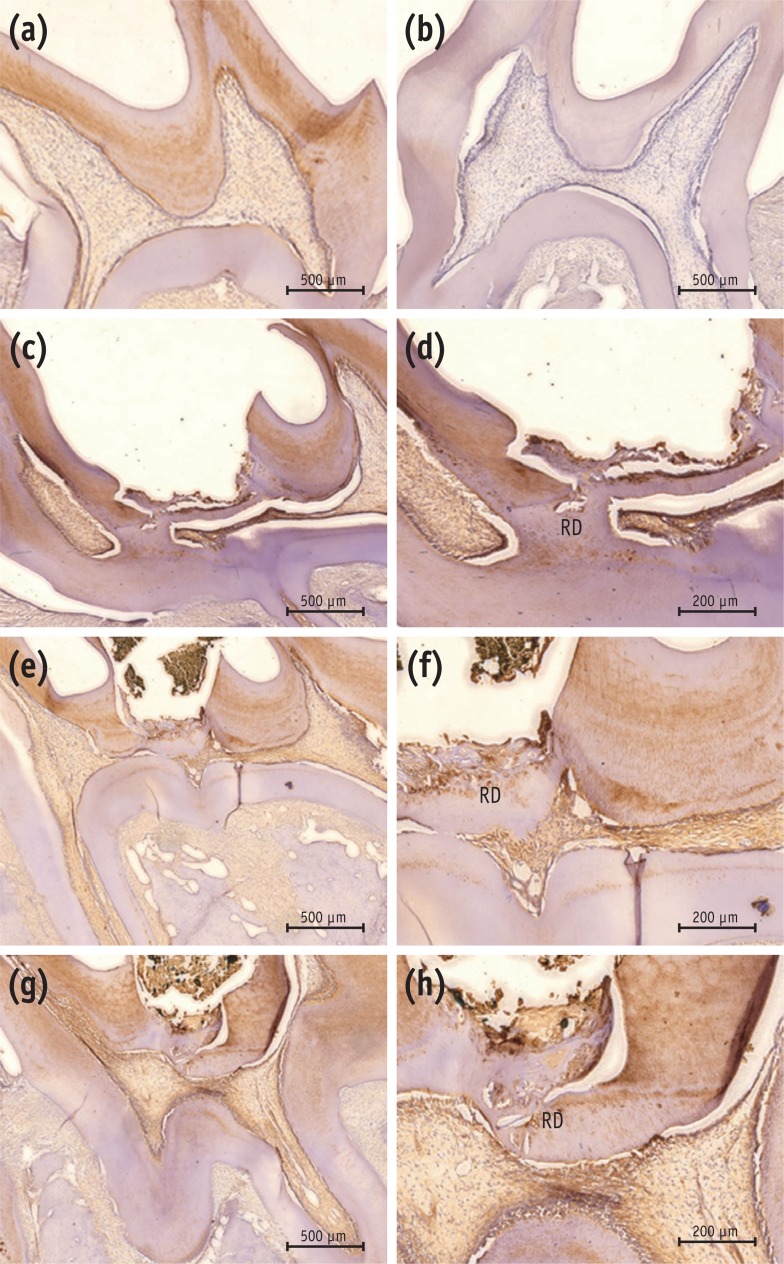
Immunohistochemical results with DSP antibody. (a) Positive control; (b) negative control; (c, d) MTA; (e, f) Biodentine; (g, h) BioAggregate. The positive control showed positive immunoreactivity to DSP in dentin and pulp (a), while the negative control was immunonegative to the DSP antibody (b). Reparative dentin from the MTA group showed strong immunoreactivity to DSP (c, d). Newly formed tissue from the Biodentine group stained lightly positive to DSP (e, f). Weakly-stained DSP immunolabeled tissue was seen in the BioAggregate group (g, h). Some immunolabeled cells were embedded within the newly formed tissue.
DSP, dentin sialoprotein; RD, reparative dentin.
Discussion
In this study, micro-CT was used to evaluate newly formed reparative dentin. Micro-CT is a noninvasive technique that presents morphological features in a nondestructive manner. It allows imaging of the interior microstructure of the subject with high spatial resolution, and its data offer detailed internal morphology. This imaging technique provides insights into the calcification patterns of dentin.2728 On micro-CT, the hard tissue barrier observed in the MTA group was thicker than that seen in the BioAggregate group. When compared with the MTA group, the Biodentine group showed an irregular, heterogeneous distribution of mineralization nodules within a uniform thickness of hard tissue barrier. In the BioAggregate group, considerable reparative dentin with relatively lower mineral density than dentin was observed. All these findings suggest that MTA, Biodentine, and BioAggregate have a dentinogenic capacity, but MTA and Biodentine have superior dentinogenic effects, when compared with BioAggregate.
Current results showed that MTA and the two calcium silicate-based materials showed favorable outcomes as a direct pulp capping material in rodent model. Some studies have reported that the pulpal response to capping materials after direct capping is affected by bacterial microleakage, which has an inhibitory effect.293031 In the present study, acute inflammatory responses and necrosis were not observed in the experimental groups. This can be explained by the fact that MTA, Biodentine, and BioAggregate all have excellent sealing ability, which prevents microleakage and pulpal inflammation and thus provides a predictable barrier.
Newly formed dentin in the MTA group had the characteristics of homogeneous reparative dentin. Specimens of Biodentine had engulfed cellular inclusions within newly formed hard tissue, and this could have been the result of the rapid initial disorganized formation of the reparative dentin. The exact mechanism by which MTA formed the dentin bridge is not completely understood. Pulp capping with MTA induced cytological and functional changes in the pulp cells, leading to the formation of fibrodentin and reparative dentin.30 MTA contains calcium oxide, which forms calcium hydroxide when mixed with water. The reaction of calcium hydroxide and the carbon dioxide from pulp tissue produced calcite crystals.32 Seux et al. demonstrated the role of calcite crystals and fibronectin as an initiating step in the formation of a hard tissue barrier.33 Laurent et al. reported that Biodentine induced an early form of reparative dentin synthesis owing to a modulation of transforming growth factor beta-1 secretion by pulp cells.34 These authors showed that Biodentine might promote the mineralization process, as shown with MTA-based cements. Tricalcium silicate, which is one of the main components of Biodentine and BioAggregate, might be associated with the stimulation of cell proliferation and differentiation.17343536
Previous studies have shown that BioAggregate is nontoxic to osteoblasts and human periodontal ligament fibroblasts and is able to induce mineralization and odontoblastic differentiation-associated gene expression in human dental pulp cells.22373839 Because of the absence of aluminum in its chemical composition, BioAggregate has fewer negative effects on the inflammatory cell response.40 In this study, we did not observe any acute inflammation or necrosis in the BioAggregate specimens. However, when compared with MTA, BioAggregate differed significantly with respect to forming a dentin bridge. Because BioAggregate differs from MTA in containing tantalum oxide instead of bismuth oxide as a radiopacifier, it may be important to further evaluate the differences between bismuth oxide and tantalum oxide in forming dentin bridges.
Vital pulp therapy requires three types of healing processes: (1) rapid formation of a hard tissue bridge to protect the pulp from other stimuli, (2) formation of a barrier that prevents secondary pulp infections, and (3) induction of hard tissue formation at the interface to avoid obliteration of the pulp.41 Above all, compact hard tissue formation without bacterial invasion is the crucial key to the success of the vital pulp therapy. We observed that MTA, Biodentine, and BioAggregate induced adequate hard tissue formation that would preserve the integrity of the pulp. All three materials formed a tight barrier and might be associated with stimulating dentinogenesis.
DSP protein was specifically found in odontoblasts and the dentinal matrix, and its expression was evaluated for dentinogenesis. DSP is synthesized as a precursor dentin sialophosphoprotein and is synthesized by terminally differentiated odontoblasts. A previous study showed positive immunostaining for DSP in the reparative dentin induced by MTA and Biodentine.36 In another study, Min et al. demonstrated that MTA-induced reparative dentin showed greater DSP immunostaining than did the calcium hydroxide groups.42 We observed higher levels of DSP expression in the MTA-treated pulp tissue than in the tissue treated with Biodentine or BioAggregate. Although, treatments with Biodentine or BioAggregate formed comparable reparative dentin, they showed weaker DSP immunostaining than did the MTA group. Some embedded immunolabeled cells were observed within the newly formed tissue in the Biodentine and BioAggregate groups. It appeared that the characteristics of newly formed reparative dentin with Biodentine and BioAggregate treatment were closer to osteodentin. These findings indicate that MTA, Biodentine, and BioAggregate induced new reparative dentin and that MTA induced dentin with better characteristics. However, further studies will be needed to demonstrate the exact mechanism and characteristics of reparative dentin induced by these three materials.
Conclusions
The results of this study suggested that Biodentine and BioAggregate might provide an optimal environment for pulp healing and repair and were comparable to MTA. Although there were some differences in the thickness and morphology of the new hard tissue, all three materials showed acceptable biocompatibility. Based on these results, calcium silicate-based materials induced favorable effects on the reparative process during vital pulp therapy and could be considered as alternatives to MTA. Further long-term studies are required for thorough evaluation of the pulpal response to these materials.
Acknowledgement
This study was supported by a Chonnam National University (2013) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2011-0030121).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
