Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials
Article information
Abstract
Objectives
The aim of this study was to evaluate the cytotoxicity, setting time and compressive strength of MTA and two novel tricalcium silicate-based endodontic materials, Bioaggregate (BA) and Biodentine (BD).
Materials and Methods
Cytotoxicity was evaluated by using a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-((phenylamino)carbonyl)-2H-tetrazolium hydroxide (XTT) assay. Measurements of 9 heavy metals (arsenic, cadmium, chromium, copper, iron, lead, manganese, nickel, and zinc) were performed by inductively coupled plasma-mass spectrometry (ICP-MS) of leachates obtained by soaking the materials in distilled water. Setting time and compressive strength tests were performed following ISO requirements.
Results
BA had comparable cell viability to MTA, whereas the cell viability of BD was significantly lower than that of MTA. The ICP-MS analysis revealed that BD released significantly higher amount of 5 heavy metals (arsenic, copper, iron, manganese, and zinc) than MTA and BA. The setting time of BD was significantly shorter than that of MTA and BA, and the compressive strength of BA was significantly lower than that of MTA and BD.
Conclusions
BA and BD were biocompatible, and they did not show any cytotoxic effects on human periodontal ligament fibroblasts. BA showed comparable cytotoxicity to MTA but inferior physical properties. BD had somewhat higher cytotoxicity but superior physical properties than MTA.
Introduction
Although there are several properties of an ideal root-end filling material, three characteristics are very crucial. The root-end filling material should be biocompatible with the periodontium, should provide a good apical seal, and it should be easy to handle.1,2,3 Many materials have been used as root-end filling materials, including amalgam, Intermediate Restorative Material (IRM), Super-EBA and mineral trioxide aggregate (MTA). MTA has the least cytotoxicity and leakage compared with other materials, and has been proven to induce osteogenesis and cementogenesis.4,5,6,7 Despite its favorable properties, MTA has some drawbacks. It has long setting time, contains heavy metals and has potential for tooth discoloration.8,9,10,11,12,13
Endodontic materials, Bioaggregate (BA, Innovative Bioceramix, Vancouver, BC, Canada) and Bioaggregate (BA, Septodont, Saint-Maur-des-Fosses, France), based on tricalcium silicate have become available recently. These materials are recommended for retrograde root filling, perforation repair and vital pulp therapy.14,15 BA contains tricalcium silicate, dicalcium silicate, calcium phosphate monobasic, hydroxyapatite, amorphous silicon dioxide and tantalum oxide. The composition of BA is similar to that of MTA, but the major difference between MTA and BA is that BA contains tantalum oxide as a radiopacifier and has no aluminum content.16 BA has an antibacterial effect and biocompatibility similar to that of MTA on primary human mesenchymal cells, osteoblasts, human pulp fibroblasts and human periodontal ligament (hPDL) fibroblasts.17,18,19,20,21
BD is composed of a powder containing tricalcium silicate, dicalcium silicate, calcium carbonate, calcium oxide and zirconium oxide as a radiopacifier and a liquid containing calcium chloride and water soluble polymer.15 BD has improved physical properties, biocompatibility with cultured pulp cells, and an ability to induce reparative dentin synthesis in pulp capping situation and odontogenic differentiation of human dental pulp cells.22,23,24,25
The manufacturer recommends the use of BD in periradicular surgeries, similar to that of MTA. However, there are no reports describing the cytotoxicity of BD on hPDL fibroblasts compared to that of MTA and BA, or the possible effects of the release of heavy metals on cytotoxicity. The aim of this study is to assess the cytotoxicity of three tricalcium silicate-based endodontic materials (MTA, BA, and BD) by evaluating the cell viability of hPDL fibroblasts, to evaluate the concentration of 9 heavy metals (arsenic, cadmium, chromium, copper, iron, lead, manganese, nickel, and zinc) leached out in distilled water, and finally, to examine the setting time and compressive strength of these three endodontic materials.
Materials and methods
Cell culture and material preparation
The hPDL fibroblasts were maintained in Dulbecco's Modified Eagle's Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, JR Scientific, Inc., Woodland, CA, USA) and 1% antibiotics (Penicillin/Streptomycin, Gibco) in a humidified incubator (MCO-18AIC, Sanyo, Osaka, Japan) with 5% CO2 at 37℃. Human PDL fibroblasts from the third to eighth passages were used for this study.
Three materials were prepared: BA, BD, and MTA. The test materials were mixed according to the manufacturer's instructions and placed into a sterile cylindrical polyethylene tube (5 mm in diameter and 3 mm in height). The excess flash was removed. Six specimens of each material were fabricated. Samples were placed in a cabinet at 37℃ and relative humidity of 95 to 100%. After 24 hours, the disc-shaped specimens were sterilized with ethylene oxide gas.
Six specimens per test materials were placed individually in wells of sterile 48-well culture plate and then 1 mL DMEM with 10% fetal bovine serum and 1% antibiotics were added. The plates with the specimens were placed in the humidified incubator at 37℃ for 24 hours to obtain the extracts. This extract comprised the culture medium containing the products released by BA, BD, and MTA. The plate was maintained for 24 hours and the extracted DMEM was harvested and filtered for sterilization.
The cells were seeded in 96-well plates at a density of 1.0 × 104 cells per well and allowed to attach for 24 hours. After the cells were attached to the plate, 100 µL of the material extract was added to each well. The hPDL fibroblasts and 100 µL of DMEM supplemented with 10% fetal bovine serum and 1% antibiotics were used as a positive control in addition to the BA, BD, and MTA groups. The cells exposed to the extracts were incubated for 1, 3, and 7 days and XTT assay was performed at each time point.
XTT assay
Cell viability was determined by the ability of the cells to cleave the tetrazolium salt (2,3-bis (2-methoxy-4-nitro-5-((sulfenylamino) carbonyl)-2H-tetrazolium-hydroxide), XTT) to a formazan dye. The cleavage of the tetrazolium salt into the water-soluble formazan is performed by the succinate-tetrazolium reductase system, which belongs to the respiratory chain of the mitochondria and is active only in the viable cells. The amount of the formazan dye is directly proportional to the number of living cells.
Ten microliters of the XTT reagent, Ez-Cytox (Daeil Lab Service Co.,Ltd., Seoul, Korea) was added to the well and incubated at 37℃ for 2 hours. The UV absorbance was measured by using a multiwall spectrophotometer (ELx800UV, Bio-Tek Instruments, Winooski, VT, USA) at the primary wavelength of 450 nm and reference of 630 nm. The relative cell viability of the test material was calculated using the following equation:

Evaluation of 9 heavy metals release in distilled water
MTA, BA and BD were mixed and placed in a sterile cylindrical polyethylene tube (15 mm in diameter and 2 mm in height). The materials were cured for 24 hours at 37℃. The materials were placed in 10 mL distilled water for 7 days in an incubator at 37℃. After 7 days, the specimens were removed, and the solutions were filtered to remove the crystalline precipitate. The concentration of 9 heavy metals (arsenic, cadmium, chromium, copper, iron, lead, manganese, nickel, and zinc) in the extracts was examined using inductively coupled plasma-mass spectrometry (ICP-MS, Perkin Elmer Nexion 300X, Perkin Elmer, Ontario, Canada). The measuring procedure was performed using ICP-MS with 4 repeats. The significance of the difference in leachable heavy metal contents among BA, BD and MTA was evaluated.
Setting time and compressive strength
The setting time of the test materials was determined by using the method recommended by the ISO 9917-1.26 The setting time was measured by using a Vicat apparatus (Humboldt Mfg. Co., Schiller Park, IL, USA). The Vicat indenter was 400 ± 5 g in weight and included a needle with a flat end, 1.0 ± 0.1 mm in diameter. The test materials were mixed and placed in a cylindrical stainless steel mold (10 mm in diameter and 5 mm in height). The assembly was placed in a cabinet at 37℃ and relative humidity of 95 to 100%, 2 minutes after the start of mixing. Ninety seconds after mixing, the indenter needle was lowered vertically onto the surface of the test material and allowed to remain there for 5 seconds. This procedure was repeated every 5 minutes until the needle failed to make a complete circular indentation in the test material. The test was repeated 10 times for each material.
The compressive strength of the test materials was determined by using the method recommended by the ISO 9917-1.26 Each material was mixed and placed in a split stainless steel mold (4 mm in diameter and 6 mm in height) within 2 minutes after the start of mixing. The complete assembly was transferred to a cabinet maintained at 37℃ for 6 hours. The specimens were removed from the molds and checked visually for any air-voids or chipped edges. All defective specimens were discarded, and 10 acceptable samples were prepared for each test material at each time interval. The specimens were immersed in distilled water for 24 hours, 3 days, and 7 days and maintained at 37℃. The compressive strengths were then measured by using a universal testing machine at a crosshead speed of 1.0 mm/min (Model GB/4302, Instron Corp, High Wycombe, Bucks, UK).
The maximum load required to fracture each specimen was determined. The compressive strength was calculated in megapascals (MPa) by using the following formula: C = 4P/D2, where P is the applied force (N) and D is the diameter (mm) of the specimen. The compressive strength of all specimens was recorded in MPa.
Statistical analysis
Two-way analysis of variance (ANOVA) followed by Tukey post hoc test was used to determine statistically significant differences in cell viability and compressive strength according to the cultivation time and the test materials. One-way ANOVA followed by the Tukey post hoc test was used to test the differences in setting time and concentration of 9 heavy metals. A p-value < 0.05 was considered statistically significant.
Results
The cell viability of hPDL fibroblasts after culturing in the extracts from BA, BD, and MTA for 1, 3, and 7 days is shown in Figure 1. The viability of cells exposed to the extracts from BA, BD and MTA was not significantly different compared to the DMEM control. When tested after 1 day and 7 days, there was no significant difference in the cell viability among the materials. At 3 days, the cell viability of BD was significantly lower than that of BA and MTA (p < 0.05). The cell viability of BA was not significantly different from that of MTA at all time points. There was no significant difference in the cell viability between BA and BD at 1 day and 7 days. With regard to the effect of time, there was no definite relationship between the cell viability of MTA and the cultivation time. There was a significant increase in the cell viability of BA after 3 days (p < 0.05), and then it decreased significantly after 7 days (p < 0.05). There was a significant increase in the cell viability of BD after 7 days compared to that after 3 days (p < 0.05).
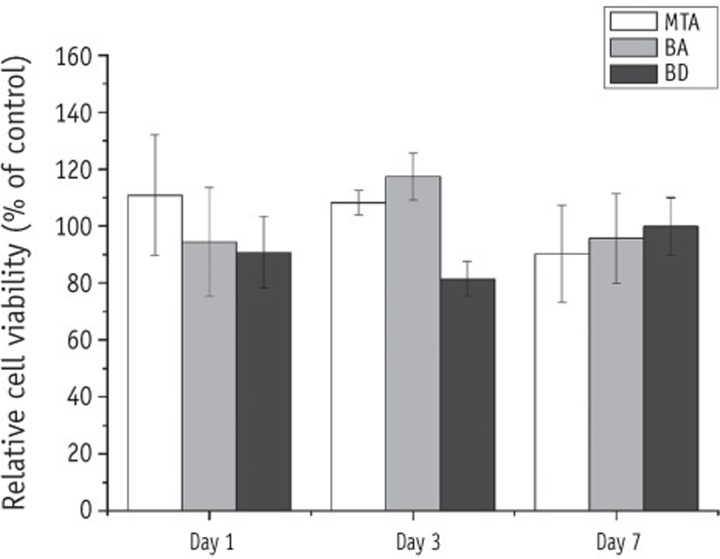
The cytotoxic effects of test material extracts on hPDL fibroblasts. The percentage of cell viability in the control group (media only group) represented 100%. There was no significantly different compared to the DMEM control. MTA, mineral trioxide aggregate; BA, Bioaggregate; BD, Biodentine.
The results for the concentration of 9 heavy metals leached out in distilled water are shown in Table 1. The levels of released arsenic, copper, iron, manganese and zinc were significantly higher in BD (p < 0.05). In all the test materials, none of the released heavy metals exceeded 0.1 ppm, except for iron. There were significant differences in the setting time among the test groups (p < 0.05, Table 2). The setting time of BD was significantly shorter than that of MTA and BA (p < 0.05). BA needed considerably more time to set than the other materials. As shown in Table 3, the compressive strength of BD was significantly higher than that of MTA and BA at all time intervals (p < 0.05). There were no significant changes in the compressive strength of BD with time. The compressive strength of MTA increased significantly with time. The compressive strength of BA was significantly lower than that of MTA and BD at all time points (p < 0.05).
Discussion
As mentioned earlier, an ideal root end filling material should be biocompatible with the surrounding host tissues. Therefore, we chose hPDL fibroblasts for this in vitro study to mimic the actual clinical situation. In this study, although the cell viability of BD was significantly lower than that of MTA (p < 0.05) at 3 days, the cell viability of BA, BD and MTA was no significantly different compared to the DMEM control (p < 0.05), suggesting that all three materials do not have a cytotoxic effect on hPDL fibroblasts.
There was no significant difference between the viability of cells exposed to extracts from BA and that of cells exposed to extracts from MTA. This result is in agreement with previous studies, which have shown that the cytotoxicity of BA was comparable to that of MTA.18,19,20,21 This could probably be attributed to the fact that chemical composition of BA is similar to that of MTA. Also, BA does not contain aluminum.16 Until now, there have been no studies comparing the cytotoxicity of BA and BD. In the present study, we found no significant difference in the cytotoxicity between these two materials.
When BA, BD and MTA were used as root-end filling materials, toxic elements leached into the surrounding environment.27 To determine the amount of leachable heavy metals, we used ICP-MS to evaluate the release, and not the presence of 9 heavy metals (arsenic, cadmium, chromium, copper, iron, lead, manganese, nickel, and zinc) in the test materials. The overall concentration of heavy metals leached out in distilled water did not exceed 0.1 ppm in all materials, except for iron (Table 2). BD released significantly higher levels of 5 released heavy metals (arsenic, copper, iron, manganese and zinc) than MTA and BA. It is likely that the release of these heavy metals from BD will have an unfavorable effect on its cytotoxicity.
In a previous study, it was reported that the leachable arsenic, chromium and lead contents were lower than 1 mg/kg, but there is no mention of whether the experimental procedures were repeated.28 The results of heavy metal analysis tend to vary widely.29 Therefore, in this study the measuring procedure was performed using ICP-MS with 4 repeats, and the significance of differences in leachable heavy metal contents among BA, BD and MTA was evaluated. Copper, iron and manganese can cause discoloration of the teeth. These elements have free electrons that are easily excited and show intensive colors when in their oxide forms.30 It has been reported that the amounts of iron oxide were significantly lower in WMTAs than GMTAs.31 The higher amounts of copper, iron and manganese released by BD could cause discoloration of the teeth. However, there is no prior report related to the discoloration of teeth after the use of BD. Further studies evaluating the tooth discoloration caused by MTA, BA and BD are needed.
In the current study, the setting time of BD was about 15 minutes, which was significantly shorter than that of the other two materials (p < 0.05). The shorter setting time of BD is due to calcium carbonate and calcium chloride.32,33 Calcium carbonate is a filler component that is often used as a hydration accelerator. Bioactive bone cement composed of tricalcium silicate and calcium carbonate showed reduced setting time and enhanced mechanical strength as compared to pure tricalcium silicate cement.33 Calcium chloride is a component of the Biodentine liquid. The % elemental analysis of the liquids performed by energy dispersive X-ray fluorescence (EDXRF) showed that the liquid contains 23.6% of calcium, 34.7% of chlorine and 20.9% of water, indicating that there is a high level of calcium chloride in the liquid.28 Addition of calcium chloride as an accelerant to MTA has been reported.34 Wang et al. have shown that the addition of calcium chloride decreased the setting time and increased the compressive strength of tricalcium silicate cement.32 The setting time of BA was significantly longer than that of MTA (p < 0.05). The manufacturers' recommended a 0.33 water-to-powder ratio of MTA and a 0.38 water-to-powder ratio of BA. Both MTA and BA were mixed with distilled water without any admixture.
According to Torabinejad et al., the compressive strength of MTA was 40 MPa after 24 hours and 67.3 MPa after 3 weeks.8 Likewise, in the present study, the compressive strength of MTA increased with time. The compressive strength values of BA showed a slight increase with time. Unlike MTA and BA, the compressive strength of BD did not increase with time. Instead, it had the highest compressive strength among the three materials after 24 hours. The compressive strength of BA was significantly lower than that of MTA and BD (p < 0.05). The strength of cements depends primarily on the water-to-powder ratio.35 The high water-to-powder ratio of BA seems to have contributed to its low compressive strength. The compressive strength of BD was significantly higher than that of MTA and BA (p < 0.05). The current results are in agreement with the work reported by Grech et al. where the average compressive strength of BA was 16.34 MPa and that of BD was 67.18 MPa after 28 days.22
Clinically, root-end filling materials do not bear direct pressure; however, materials used for pulp capping or perforation in the gingival third area bear occlusal pressure. Therefore, it is important to consider the compressive strength of materials placed on the occlusal surfaces.8
Conclusions
Although we need to obtain more information about the cytotoxicity and physical properties of BA and BD, based on the results of this study, we can suggest that BA and BD might not cause any cytotoxic effect on the surrounding environment. It seems that BD showed adequate physical properties for its use as a filling material for vital pulp therapy or as a dentine substitute, but it was more cytotoxic to hPDL fibroblasts than MTA. Also, BD might release higher amounts of heavy metals than MTA and BA. BD may cause a tooth discoloration, because it contains large amount of iron. Conversely, BA showed inferior physical properties but comparable cytotoxicity to MTA. It could be used as a root-end filling material. Further studies evaluating the in vivo biocompatibility and tooth discoloration caused by MTA, BA and BD are needed.
Acknowledgement
The first 2 authors contributed equally to this work. The authors deny any conflicts of interest related to this study. This study was supported by a grant (CRI 13029-1) Chonnam national university hospital research institute of clinical medicine
Notes
No potential conflict of interest relevant to this article was reported.


