References
1. Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod 2004. 30785–787.
2. Park JH. The effect of solvent action of sodium hypochlorite solution on pulp tissue. J Korean Acad Conserv Dent 1982. 8115–122.
3. Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifacio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod 1999. 25167–171.
4. Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod 1998. 24472–476.
5. Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod 1994. 20276–278.
6. Ferguson JW, Hatton JF, Gillespie MJ. Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J Endod 2002. 2868–71.
7. White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod 1997. 23229–231.
8. Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod 1994. 20276–278.
9. Ohara P, Torabinejad M, Kettering JD. Antibacterial effects of various endodontic irrigants on selected anaerobic bacteria. Endod Dent Traumatol 1993. 995–100.
10. Delany GM, Patterson SS, Miller CH, Newton CW. The effect of chlorhexidine gluconate irrigation on the root canal flora of freshly extracted nectoric teeth. Oral Surg Oral Med Oral Pathol 1982. 53518–523.
11. Kim HJ, Park SH, Cho KM, Kim JW. Evaluation of time-dependent antimicrobial effect of sodium duchloroisocyanurate (NaDCC) on E. faecalis in the root canal. J Korean Acad Conserv Dent 2007. 32121–129.
12. Lee JK, Baik JE, Yun CH, Lee K, Han SH, Lee W, et al. Chlorhexidine gluconate attenuates the ability of lipoteichoic acid from Enterococcus faecalis to stimulate toll-like receptor 2. J Endod 2009. 35212–215.
13. Rosenthal S, Spaångberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004. 98(4)488–492.
14. Basrani BR, Manek S, Sodhi RN, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod 2007. 33(8)966–969.
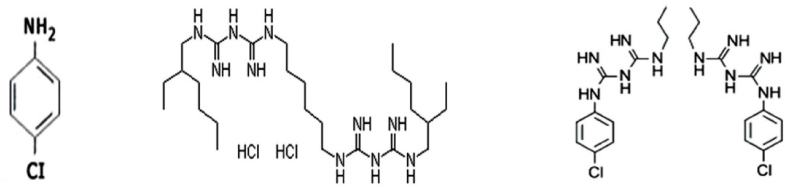
15. Chhabra RS, Huff JE, Haseman JK, Elwell MR, Peters AC. Carcinogenicity of p-chloroaniline in rat and mice. Food Chem Toxicol 1991. 29119–124.
16. Burkhardt-Holm P, Oulmi Y, Schroeder A, Storch V, Braunbeck T. Toxicity of 4-chloroaniline in early life stages if Zebrafish(Danio Rerio): II. Cytopathology and regeneration of liver and gills after prolonged exposure to waterborne 4 chloaniline. Arch Environ Contam Toxicol 1999. 3785–102.
17. Bui TB, Baumgartner JC, Mitchell JC. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod 2008. 34181–185.
18. Zehnder M. Root canal irrigants. J Endod 2006. 32389–398.
19. Rasimick B, Nekich M, Hladek M, Musikant B, Beutch A. Interaction between chlorhexidine digluconate and EDTA. J Endod 2008. 341521–1523.
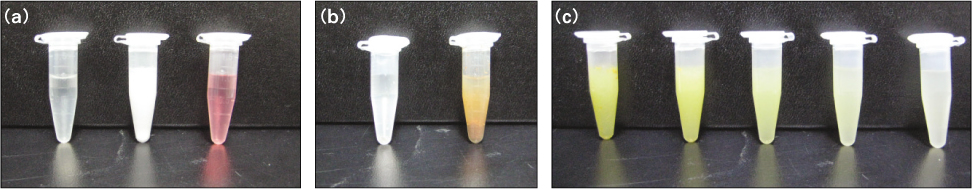
20. Choi MS, Park SH, Cho KM, Kim JW. The comparison of different canal irrigation methods to prevent reaction precipitate of sodium hypochlorite and chlorhexidine. J Korean Acad Conserv Dent 2010. 3580–87.
21. McDonnell , Russell AD. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev 1999. 12147–179.
22. Zorko M, Jerala R. Alexidine and chlorhexidine bind to lipopolysaccharide and lipoteichoic acid and prevent cell activation by antibiotics. J Antimicrob Chemother 2008. 62730–737.
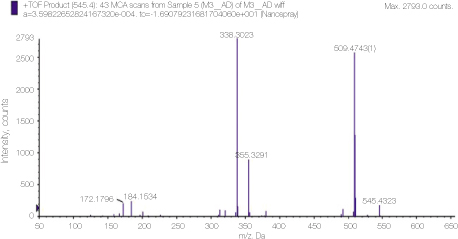
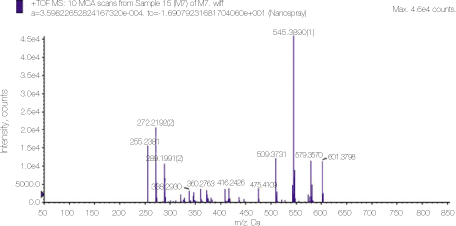
23. Prestidge CA, Barnes TJ, Skinner W. Time-of-flight secondary-ion mass spectrometry for the surface characterization of solid-state pharmaceuticals. J Pharm Pharmacol 2007. 59(2)251–259.
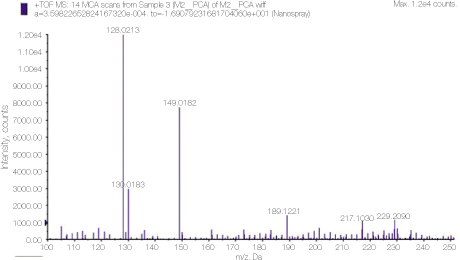
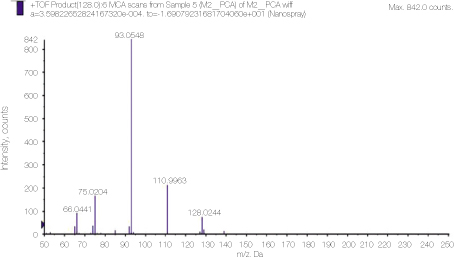
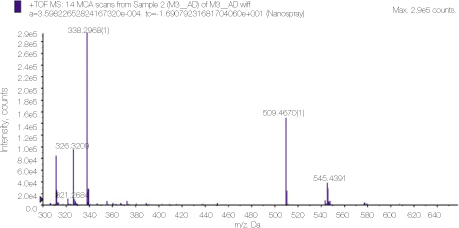
24. Thomas J, Sem D. An in vitro spectroscopic analysis to determine whether para-chloroaniline is produced from mixing sodium hypochlorite and chlorhexidine. J Endod 2010. 36315–317.
25. Basrani BR, Manek S, Mathers D, Fillery E, Sodhi RN. Determination of 4-chloroaniline and its derivatives formed in the interaction of sodium hypochlorite and chlorhexidine by using gas chromatography. J Endod 2010. 36(2)312–314.
26. Barbin LE, Saquy PC, Guedes DF, Sousa-Neto MD, Estrela C, Pécora JD. Determination of para-chloroaniline and reactive oxygen species in chlorhexidine and chlorhexidine associated with calcium hydroxide. J Endod 2008. 34(12)1508–1514.
27. Weatherford TW 3rd, Finn SB, Jamison HC. Effects of an alexidine mouthwash on dental plaque and gingivitis in humans over a six-month period. J Am Dent Assoc 1977. 94528–536.
28. Spolsky VW, Forsythe AB. Effects of alexidine.2HCL mouthwash on plaque and gingivitis after six months. J Dent Res 1977. 56(11)1349–1358.
29. Roberts WR, Addy M. Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine. I. Effect on plaque accumulation and salivary bacteria. J Clin Periodontol 1981. 8(3)213–219.
30. Addy M, Roberts WR. Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine. II. Clinical and in vitro staining properties. J Clin Periodontol 1981. 8(3)220–230.
31. Chawner JA, Gilbert P. A comparative study of the bactericidal and growth inhibitory activities of the bis-biguanides alexidine and chlorhexidine. J Appl Bacteriol 1989. 66(3)243–252.
32. Baker PJ, Coburn RA, Genco RJ, Evans RT. Structural determinants of activity of chlorhexidine and alkyl bis-biguanides against the human oral flora. J Dent Res 1987. 66(6)1099–1106.
33. Yip KW, Ito E, Mao X, Au PY, Hedley DW, Mocanu JD, et al. Potential use of alexidine dihydrochloride as an apoptosis-promoting anticancer agent. Mol Cancer Ther 2006. 5(9)2234–2240.