The sustaining effect of three polymers on the release of chlorhexidine from a controlled release drug device for root canal disinfection
Article information
Abstract
The aim of this in vitro study was to evaluate the suitability of using chitosan, poly (lactide-co-glycolide) (PLGA), and polymethyl methacrylate (PMMA) to control the release of chlorhexidine digluconate (CHX) from a prototype of controlled release drug device (CRD) for root canal disinfection. Four different prototypes with different formulations were prepared. Group A (n = 12); The device (absorbent paper point) was loaded with CHX as control. Group B (n = 12); same as group A, but the device was coated with chitosan. In Groups C and D, the device was treated in the same way as group A and then coated three times with 5% PMMA (Group C, n = 12), or coated three times with 3% PLGA (Group D, n = 12). The devices were randomly allocated to experimental groups of 12 each.
All CRD prototypes were soaked in 3 mL distilled water. The concentrations of CHX were determined using a UV spectrophotometer. The surface characteristics of each prototype were observed using a scanning electron microscope.
The result showed that release rate of CHX was the greatest in the non-coated group, followed by the chitosan-coated group, the PLGA-coated group, and the PMMA-coated group (P < 0.05). Pores were observed on the surface of the prototypes that were coated with PLGA and PMMA. When the pore size was smaller, the release rate was lower. This data indicate that polymer coating can control the release rate of CHX from the CRD prototypes.
I. Introduction
Complete debridement and effective disinfection of the root canal space are considered essential for predictable long-term success of endodontic treatment1). However, instrumentation and irrigation is not always effective in eliminating a therapy-resistant microflora in the root canal system1-3). Calcium hydroxide has proven to be an excellent antimicrobial agent for intracanal dressing in the treatment of infected root canals4,5). However, it is known to be less effective against Enterococcus faeclais, Actinomyces and Candida that are frequently isolated in persistent/infected root canals6). The antimicrobial efficacy of calcium hydroxide to affect microorganisms entrenched in the dentinal tubules is also questionable7). Therefore, alternative medicaments should be explored that would maximize microbial eradication when used as intracanal dressings.
Chlorhexidine is effective against a wide variety of Gram-positive and Gram-negative organisms, as well as fungi. Recent studies have shown that the antimicrobial effect of chlorhexidine digluconate (CHX) was equal to that of the conventional irrigants and medicaments8-11). In addition, it is retained by the dentinal hard tissues and thus has a substantive antimicrobial action12-14). It was also suggested as an effective irrigant to prevent root canal reinfection due to coronal leakage15). However, in order to achieve long-term substantive antimicrobial effect, the infected root dentin must be exposed to CHX for a longer time than that afforded by irrigation16,17).
A number of studies have shown that a controlled release drug (CRD) device with water-permeable polymer could effectively sustain the release of CHX from the CRD16,17). However, because of a strong, positive charge of chlorhexidine and its high binding affinity, the development of suitable drug carriers for sustained release of CHX still remains a challenge.
Chitosan, poly (lactide-co-glycolide) (PLGA), and polymethyl methacrylate (PMMA), are well known polymers as controlled drug release carrier. Miyazaki et al. observed the sustaining effect of chitosan on the release of water insoluble indomethacin from granules18). A sustained plateau level of indometacin was obtained for drug/chitosan granules (1 : 2 mixtures) versus a sharp peak for conventional commercial capsules in a rabbit model. PLGA is one of the best-known biodegradable polymers. It is hydrolyzed without enzymes and metabolized by the body19). Moreover, the degradation rate of PLGA can be regulated by changing its molecular weight, chemical composition, and crystal form20). PMMA has been used as denture base materials, and one recent study suggested that it could be used as a controlled drug release carrier for antibiotics, for the prevention and treatment of osteomyelitis21). Therefore, all three polymers may be promising controlled drug release carriers.
The aim of this in vitro study was to compare the sustaining effect of chitosan, PLGA, and PMMA on the release of CHX from a prototype of CRD device for root canal disinfection.
II. MATERIALS AND METHODS
1. Standard curve of CHX concentration.
CHX solution (20% wt / wt, Sigma, St. Louis, MO, USA) was diluted serially in 1:1 ratios, and the UV absorbance was measured for each dilution using a UV spectrophotometer (Shimadzu, Tokyo, Japan). The standard curve of CHX concentration versus UV absorbance was used to determine CHX concentration in the experiments.
2. Preparation of the prototype of CRD.
Absorbent paper points (Sure-Endo™, #80, Chungju, Korea) were used as core material. Four different prototypes with different formulations were prepared: group A; absorbent paper points were loaded with CHX. The paper points were immersed in 40% concentrated CHX solution obtained by drying process for 30 minutes and then dried. The 40% concentrated CHX solution was obtained by evaporating water of 20% CHX solution in an oven at 50oC until target weight was reached. Group B; after loading with CHX as in group A, the paper points were coated with an acidic aqueous 3% solution of chitosan (Texan MedTech, Kwangju, Korea) and dried. Groups C and D were treated as Group B except that the paper points were coated three times with 5% PMMA (Group C, Aldrich®, Milwaukee, WI, USA) in methylene chloride, or three times with 3% PLGA (Group D, Sigma®, St. Louis, MO, USA) in methylene chloride, respectively. For Group C and D, the CHX-loaded paper points were dip-coated with polymer solutions and dried, and this process was repeated twice. All loaded absorbent paper points were individually weighed before being coated. The ones with the range of 0.033 ± 8.43 × 10-5 g were selected, and they were randomly allocated to experimental groups of 12 each.
3. Measuring release of CHX from a prototype of CRD.
Each prototype was immersed in 3 ml of distilled water. 10µl of this solution was then sampled at predetermined times (i.e., at 3, 6, 10, 20, 30, 40 and 50 min and at 1, 2, 3, 4, 5 and 6h, and at 7days). UV absorbance was measured using a UV spectrophotometer (Shimadzu, Tokyo, Japan) to determine the concentration of released CHX from the CRD prototype.
4. Surface observations of CRD devices by SEM.
Each prototype was coated with a thin palladium-gold film, and viewed the surface characteristics under a scanning electron microscope (JEOL, TSM-6320F, Tokyo, Japan) at magnifications of 100× and 5000×.
5. Statistical analysis.
One-way ANOVA test was used to compare the release rates of CHX in each group. The significance was established at 5% level (p = 0.05).
III. Results
1. Standard curve of CHX concentration.
The average weight of CHX loaded in the paper points was 0.016g/point. If all the CHX loaded in the paper point was released into 3mL of distilled water, the concentration would have been about 0.53%. From Figure 1, we calculated that the UV absorbance of 0.53% CHX was about 1.1, which thus represented the maximum UV value.
2. Release rate of CHX from the CRD devices.
Statistically significant differences were found between the groups by one-Way ANOVA (P < 0.05). The release rate of the CHX was the greatest in the non-coated group, followed by the chitosan-coated group, 3% PLGA-coated group, and 5% PMMA-coated group (Figures 2 and 3).
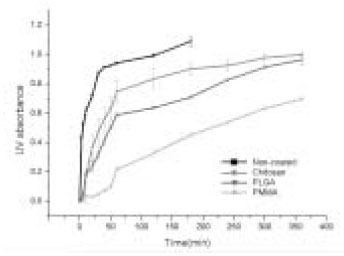
Short-term release rate of CHX after immersion of controlled release drug device in 3 ml of distilled water.
3. Surface observations of CRD devices by SEM.
Scanning electron micrographs showed the different surface characteristics of the CRD prototypes. In the non-coated group, the fiber structure of the absorbent paper point was unaffected and no surface pores were observed (Figure 4). In the polymer-coated groups, coated fiber structure was observed in all prototypes. However, the surface pores were only observed in the PMMA- and PLGA-coated groups, and the pore sizes differed between the two groups. The pore size of the PLGA-coated group is about 2 µm and larger than that of the PMMA-coated group which is <1 µm in size (Figures 5 and 6). The chitosan covered absorbent paper points did not show any pores (Figure 7).
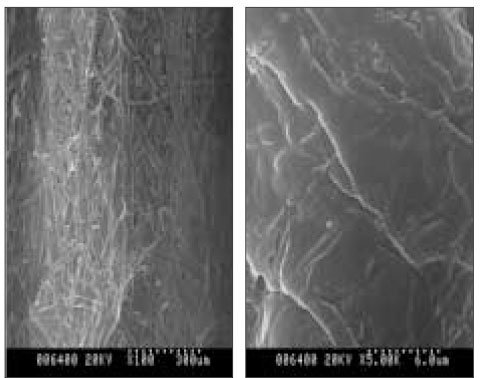
SEM images of the non-coated paper point which was loaded with CHX; (a) 100× (b) 5000×; the fiber structure of the paper point was observed without pores.
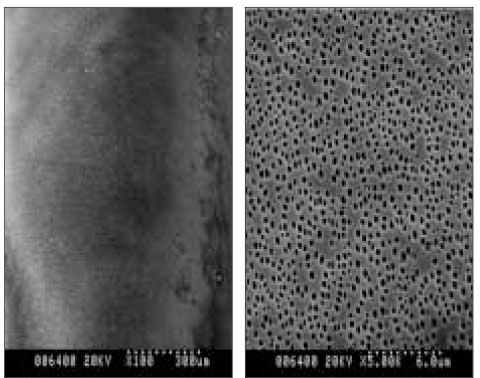
SEM images of a prototype of controlled release drug device coated with 5% PMMA three times; (a) 100×, (b) 5000×.; surface pores were observed, and all were within 1 µm.
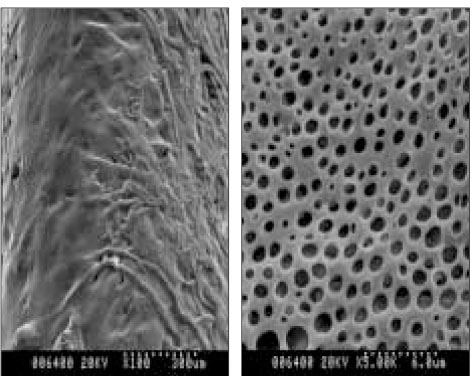
SEM images of a prototype of controlled release drug device coated with 3% PLGA three times; (a) 100×, (b) 5000×; pore sizes larger than those of the PMMA-coated group were observed.
IV. Discussion
Huang et al.22) developed the cylindrical, needle-shaped CRD prototypes with different formulations and demonstrated that the releasing rate of CRD with non-coated formulation was very fast. In contrast, the release rate of CRD with coated formulations was far more controlled.
In this study, similar results were obtained. In the non-coated group, the drug release was very fast, and all loaded CHX was released within 2h. In contrast, CHX release from the polymer-coated groups was more controlled. Chitosan was more sensitive to water and easily swollen with water and ruptured. This resulted in faster release of CHX from the chitosan-coated CRD device compared to the PLGA- and PMMA-coated groups. The CHX loaded in the paper point was released through the surface pores on the coated polymer layer. The pore size of PLGA-coated group was larger than that of PMMA-coated group and the release rate of CHX from the latter group was lower than that of the former group. Thus, the surface pore size was very important for the release rate of CHX and various release rates of CHX from CRD devices can be achieved by controlling the pore size of the coated polymer. The ideal CRD device should have the following characteristics. It should not degrade inside the root canal and it should be easily inserted into and removed from the root canal. In addition, the drug should be released continuously for a controlled time period. Heling et al16,17) developed a CRD device containing a biodegradable polymer and demonstrated that it was more effective than calcium hydroxide at disinfecting dentinal tubules. However, if used for root canal disinfection, it may not be completely degraded at the time for root filling. Any remaining fragments in the root canal may interfere with the permanent filling, and thus result in leakage.
Due to this concern, insoluble polymers were used for coating. Chitosan is insoluble at an alkaline or neutral pH23). PMMA, which has been used for denture base, is also an insoluble and nondegradable material. PLGA is a biodegradable polymer, but the degradation rate of PLGA can be controlled using the lactide to glycolide mole ratio19). Therefore, all the materials used in the present study are suitable as coatings for drug carrier for root canal disinfection. The use of absorbent paper point as core material can easily be inserted into root canals and they can be easily removed from the root canal after use.
Based on the above results, we conclude that the polymer coating can effectively control the release rate of CHX from the CRD prototypes. Further studies are needed to evaluate the antimicrobial effects and the cytotoxicity of these prototypes of CRD device.
Notes
This study was partly supported with 2003 Yonsei university research fund.


