Comparative evaluation of the biological response of conventional and resin modified glass ionomer cement on human cells: a systematic review
Article information
Abstract
This review aimed to evaluate and compare the biological response (biocompatibility and cytotoxicity) of resin modified glass ionomer cement (RMGIC) in contrast to conventional glass ionomer cement (GIC) on human cells. Articles reporting parallel and split-mouth clinical trials, randomized controlled trials, non-randomized controlled trials, prospective studies, and in vitro studies on human permanent teeth that assessed the biological response of GIC and RMGIC were included. The following electronic bibliographic databases were searched using the keywords: MEDLINE/PubMed, EBSCO, Cochrane Central Register of Controlled Trials, and Google Scholar. For the risk of bias MINORS tool and the modified scale of Animal Research: Reporting of In Vivo Experiments and Consolidated Standards of Reporting Trials were used. Initial screening identified 552 studies, of which 9 articles met the inclusion criteria and were included in the study. Different parameters such as odontoblastic changes, inflammatory response, tertiary dentin formation, presence of microorganisms, morphological changes, cell viability, number, and metabolism were used to evaluate the biological response of conventional GIC and RMGICs. Conventional GIC shows lower cytotoxicity compared to RMGIC in vital pulp therapy procedures. Further, in vivo studies and long-term clinical trials are needed to compare these observations for pulp therapy using the 2 test materials.
Trial Registration
PROSPERO Identifier: CRD42023426021
INTRODUCTION
Dental cements are crucial in dentistry, serving as foundations, protective layers, fillings, or adhesives for dental devices, with diverse options chosen based on specific needs. Their careful selection significantly impacts dental restoration success. Typically, these cements solidify by mixing powder and liquid to meet various criteria, including safeguarding tooth tissues, resisting tension and pressure, forming durable bonds, and biocompatibility and impermeability. Along with ease of use, low solubility, radiopacity, optimal working times, high resistance, suitable viscosity, and aesthetics, it is imperative for the cements to have the ability to ensure that a dental treatment accomplishes its intended purpose effectively, it should operate without causing any unwanted local or systemic effects for the patient. Instead, it should stimulate the most suitable beneficial response from cells or tissues in the particular scenario, ultimately maximizing the therapy’s practical effectiveness in clinical settings [1].
Dental cements are categorized by purpose, composition, and properties, including glass ionomer cements (GICs), introduced in 1969 by Wilson and Kent in London, which release fluoride to inhibit caries through a consistent process, acting as a fluoride reservoir for long-term effectiveness.
Extensive research has probed the biocompatibility of GICs, revealing that these cements generally fall within biocompatible parameters, although initial pulp reactions may occur, with uncertain long-term effects on pulp tissue [23]. GICs come in various forms and are set within 2–3 minutes, fully hardening in up to 48 hours [4]. Adhering to the recommended powder-liquid ratio is crucial. GICs’ unique attributes, including fluoride release, biocompatibility, direct bonding to teeth, good marginal adaptation, and dentin-like elasticity, make them versatile for dental procedures such as restorations, liners, luting, crowns, and bridges. However, they have limitations due to suboptimal mechanical properties, slow setting, high solubility, and sensitivity to moisture, restricting their use in stress-prone areas like posterior teeth [24]. Efforts to improve GICs have involved modifying glass powder formulations with elements like resin, hydroxyapatite, fibers, and nano-sized particles, with mixed effects on their properties [5].
Resin modified glass ionomer cements (RMGICs), introduced in 1991, aimed to overcome the limitations of traditional GICs by incorporating elements like hydroxyethyl methacrylate (HEMA), triethylene glycol dimethacrylate (TEGDMA) and initiators for polymerization into the basic GIC composition. This hybrid approach combines acid-base and polymerization reactions, reducing microleakage while retaining the benefits of fluoride release and tooth bonding. RMGICs are easier to handle and bond well with composite materials and can be cured on command thereby improving its handling properties and increasing acceptance among the clinicians. However, assessing the biocompatibility of these materials is crucial, especially when compared to conventional GICs, which share similar compositions and reactions [6]. The biological response of RMGIC is believed to be compromised due to the release of components like HEMA, which can have cytotoxic effects on pulp cells, posing potential risks to dental professionals and patients.
Evaluating dental material biocompatibility is crucial for patient and dental professional well-being, aiding in the selection of suitable dental cements. Some commonly used dental cements have exhibited cytotoxic effects on both soft and hard tissues, potentially affecting the long-term success of restorations [78]. The degree of cytotoxicity varies among cements, depending on their specific composition and the leachable components within them. For instance, GICs were initially thought to be toxic due to fluoride, but subsequent studies revealed other major components as the culprits [59]. Conversely, resin-based cements, including RMGICs, tend to exhibit higher cytotoxicity due to the presence of monomers like HEMA, TEGDMA, and bisphenol A-glycidyl methacrylate, which can adversely affect various oral tissues Strict adherence to recommended polymerization times is essential to mitigate these effects [310].
The diffusion of these monomers into dentin allows them to interact with pulp cells, potentially disrupting cell function, inhibiting differentiation, and reducing the formation of mineral nodules within teeth [31112]. Monomers may also induce immune responses, leading to allergies and hypersensitivity [131415]. Additionally, certain monomers like HEMA and TEGDMA have been linked to genotoxic effects, including increased micronucleus formation and chromosomal aberrations [1617]. Clinical, radiographic, and histological evaluations are critical in assessing the biocompatibility of dental materials, covering aspects like cell viability, metabolism, morphology, inflammatory responses, tissue organization, and more.
RMGIC has superior mechanical and physical properties, superior handling, and better adhesiveness as compared to conventional GIC. However, literature shows conflicting views of pulpal response towards RMGIC in deep cavities [36].
The current scientific literature lacks a systematic review directly comparing the biological response in terms of biocompatibility and cytotoxicity of conventional GIC and RMGIC when used in direct or indirect pulp capping or pulpotomy. Thus, the objective of this systematic review is to evaluate and compare the biological response (biocompatibility and cytotoxicity) of resin modified glass ionomer cement in contrast to conventional glass ionomer cement on human cells.
MATERIALS AND METHODS
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the protocol was registered in the International Prospective Register of Systematic Reviews database (PROSPERO) with registration number CRD42023426021. Also, a well-defined review question was developed by using the patient Population, Intervention, Comparison, and Outcome (PICO) framework.
Eligibility criteria
The aim of the present review is to investigate the biological response of GIC and RMGIC which are commonly used restorative materials.
The following PICO framework was developed for a systematic review.
Population: Human carious tooth/iatrogenic exposed pulp undergone Indirect or direct pulp capping or pulpotomy procedure with GIC or RMGIC or cell culture (odontoblasts, fibroblast, dental pulp stem cells or any other cells) exposed to material extracts of conventional GIC or RMGIC.
Intervention/Exposure: Treatment of human carious tooth/Iatrogenic exposure of pulp using Indirect pulp capping, direct pulp capping and pulpotomy procedure OR exposure of cell culture (odontoblasts, fibroblast, dental pulp stem cells or any other cell) to the material extract of conventional GIC (Inocid-L 30, Ketac-Fil, Ketac- Molar or any other commercially available conventional GIC).
Comparison: Treatment of human carious tooth/Iatrogenic exposure of pulp using Indirect pulp capping, Direct pulp capping and pulpotomy procedures using RMGIC or exposure of cell culture (odontoblasts, fibroblast, dental pulp stem cells or any other cell) to the material extract of RMGIC (Vivaglass, Vitremer 3M, Vitrebond or any other commercially available RMGIC).
Outcome: The main outcome is in terms of biological response to the included material. It was based on histological, clinical, and radiographic evaluation. The histological evaluation included cell viability, cellular metabolism, cell morphology, odontoblastic changes, inflammatory cell infiltration, reactionary dentin formation, presence of microorganisms, tissue disorganization, etc. Clinical parameters included the presence or absence of pain, swelling, sinus or periodontal pockets, tenderness on percussion, or any other symptoms. Radiographic parameters like changes in the periapical region, root resorption, or any other method of evaluation were included.
Review question
Among conventional GIC and RMGIC, which material is better in terms of biological response?
1. Inclusion criteria
The inclusion criteria were 1) Articles reporting parallel and split-mouth clinical trials, randomized controlled trials, non-randomized controlled trials, prospective studies, and in vitro studies on human permanent teeth; 2) Studies in which conventional GIC and RMGIC are used for direct or indirect pulp capping; 3) Studies in which cell culture (odontoblasts, fibroblast, dental pulp stem cells or any other cells) is exposed to material extracts of conventional GIC or RMGIC is used; 4) Studies in which biological response was assessed by using histological, clinical and radiographic criteria; and 5) Articles in English language or other languages where English translation is possible.
2. Exclusion criteria
The inclusion criteria were 1) Case reports, reviews and expert opinion; 2) Studies with only abstracts without the availability of full text; 3) Studies on primary teeth; and 4) Animal studies.
Information sources
The following electronic bibliographic databases were searched: MEDLINE/PubMed, PubMed Central, EBSCO, Cochrane Central Register of Controlled Trials as well as Google Scholar. Other searches included hand searches of articles, grey literature, citations as well as cross-references.
Search strategy
The following terminologies were searched: ((((((GIC) OR (Glass ionomer cement)) OR (Ketac-Molar)) OR (Ketac-Fil)) OR (Glass-Ionomer Cements)) AND ((((((Resin modified glass ionomer cement) OR (RMGIC)) OR (Light curing GIC)) OR (Vivaglass,)) OR (Vitremer 3M)) OR (Vitrebond))) AND ((((((((Biocompatibility) OR (Pulpal response)) OR (Human Odontoblast)) OR (Gingival fibroblast)) OR (Dental pulp stem cells)) OR (Cytotoxicity)) OR (Primary culture)) OR (Histopathology)) Filters: Free full text, Full text.
Selection process
The study selection was done by 2 reviewers (SS) and (RJ). During the first step, the articles from the different databases were imported with software (Mendeley), and duplicate articles were eliminated. Then the titles and abstracts of the articles were reviewed to eliminate the irrelevant articles. In the final step, the articles were filtered through the full reading of each one of them. During each step, a third reviewer (GK) could be consulted if the 2 reviewers were not able to resolve disagreement through discussion.
Data collection process and data items
Data extraction was done in 4 domains: 1) Identification of the study (type of the study, article title; journal title; authors; country of the study; language; publication year; host institution of the study); 2) Methodological characteristics (study objective or research question; sample characteristics, e.g., sample size, age, type of procedure performed, type of material used, type of cells used, different concentration of material extract, methods of evaluation, statistical analyses, etc.; 3) Main findings (Histological, clinical, and radiographic outcome); and 4) Conclusions or remarks.
Study risk of bias assessment (quality assessment)
The assessment of the risk of bias was done by 2 reviewers (RJ and SS), to analyze the methodological quality of the articles included. For the assessment of the non-randomized clinical trials, the Methodological Index for Non-Randomized Studies (MINORS) tool was used [18]. This tool has 8 items for non-comparative studies and 12 items for comparative studies. On this scale, items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal score is 16 for non-comparative studies and 24 for comparative studies.
To assess the risk of bias in the in vitro studies, the modified scale of Animal Research: Reporting of In Vivo Experiments (ARRIVE) and Consolidated Standards of Reporting Trials (CONSORT) was used (the highest score is 25, acceptability range is 18–25) [19].
RESULTS
Study selection
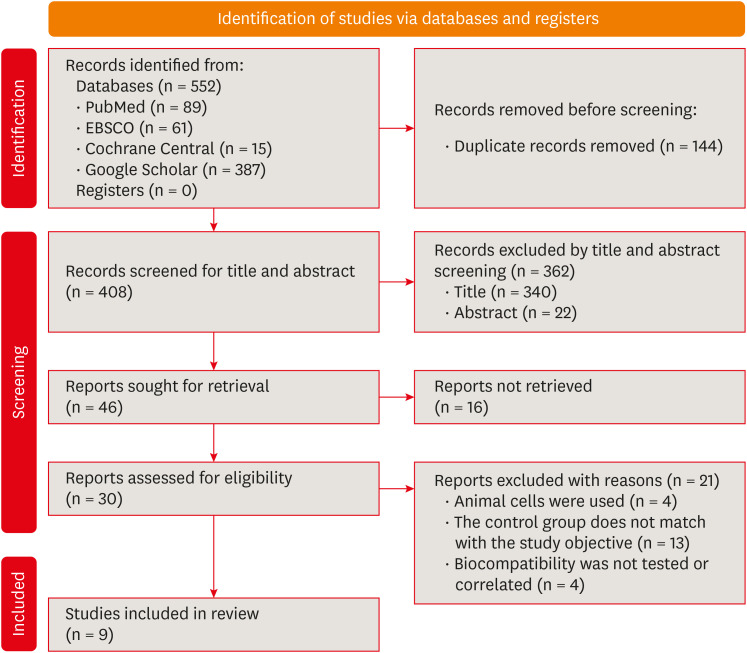
Initial screening found 537 studies, yielding 408 screened abstracts after removing duplicates. Of these, 46 full texts were considered eligible. However, 16 articles could not be retrieved in full. After a thorough assessment, 9 papers were included for review, while 21 were excluded for various reasons (refer to the PRISMA flow diagram and Figure 1 for details on excluded articles).
Study characteristics
Out of 10 included studies 3 studies were nonrandomised clinical studies whereas 6 studies were in vitro studies [202122232425262728]. Three studies assessed histological response by using premolars [202122]. Six studies used various cell lines namely human gingival fibroblast (HGF), human dental pulp fibroblast (HDPF), stem cell human exfoliated deciduous teeth (SHED), and human odontoblast cell line (MDPC-23) [232425262728]. Odontoblastic changes inflammatory response, tertiary dentin formation, presence of microorganisms, assessment of morphological changes, lactate dehydrogenase release (LDH), cell viability percentage, RNA expression for RPL13, stromal cell-derived factor-α value, interleukin (IL)-8, IL-6, Nitrito, etc. were the different parameters which were considered to assess the biological response of conventional and RMGICs. Table 1 shows the general characteristics of the studies included in the review, and Tables 2 and 3 show details of various parameters used to assess the biocompatibility and cytotoxicity of conventional and RMGIC.
Six studies have used various cell cultures (odontoblasts, fibroblast, dental pulp stem cells, or any other cell) and exposed them to the material extract of conventional GIC and resin modified GIC to assess the cytotoxicity [232425262728]. They have used various cytotoxicity tests like DNA Intercalating fluorochrome assay, Phase contrast microscopy, LDH assay, EPXMA analysis, TRIPAN blue, MTT assay, cytokine detection by enzyme-linked immunosorbent assay and reverse transcription-quantitative polymerase chain reaction, Griess methods for nitric oxide release mRNA expression and Water-Soluble Tetrazolium 1 assay. All 6 studies showed that the cytotoxic effect of conventional GIC was significantly lesser as compared to RMGICs (Table 2). Three studies have compared the histological response of pulp to RMGIC and conventional GIC in terms of inflammatory response, tissue disorganization, reactionary dentin formation, number of bacteria, odontoblastic changes at 5–7 days and 30 days follow-up [202122]. All these are recorded as no changes, mild, moderate, and severe changes. These studies have shown favorable results with conventional GIC as compared to RMGIC (Table 3).
Risk of bias (quality assessment)
The 3 non-randomized controlled clinical trials presented a score on the MINORS tool as 13 or 14 out of 16 points (Table 4), therefore they can be considered as “low- risk” of bias studies. Concerning the in vitro studies, 2 studies showed a moderate risk of bias whereas the 5 other studies presented a low risk of bias according to the modified ARRIVE and CONSORT scale (Table 5).
DISCUSSION
The present systematic review exhibits information, based on scientific evidence, related to the biological response of conventional GIC compared to RMGIC. The aim of this study was to analyze the toxicity of RMGIC in contrast to conventional GIC on the human carious tooth or exposed pulp undergoing direct or indirect pulp capping or human cell culture exposed to extracts of the test materials.
Numerous factors, including the heat damage caused during cavity preparation, the existence of bacteria, their metabolites as well as the toxicity of substances released from dental materials can induce pulp damage [20]. Applying dental materials in cavities with thin remaining dentin thickness (RDT) tests the biologic behavior and response of both the dental material and the pulp dentin complex. Research has shown that maintaining a 0.5 mm layer of dentin between the pulp and the restorative material effectively shields the pulpal tissue from the harmful effects associated with the materials used. Factors such as wide dentinal tubules and increased moisture levels can facilitate the penetration of potentially harmful chemical residues from resin-based dental products including bonding agents, which could pose risks to the health of the nearby pulp tissue. Therefore, the use of a biocompatible liner in cases of deep dentinal caries is advisable [242930].
Resin-based dental materials contain components having cytopathic properties [31]. The primary resin monomer used in bonding agents RMGIC is HEMA, whose potential for toxicity is well known. HEMA induces DNA strand breakage, leading to apoptosis and genotoxic effects in culture media [32]. When ethanol elution was employed to extract HEMA from RMGIC, it notably led to a reduction in the material’s cytotoxicity to pulp cells. Moreover, HEMA hinders the secretion of inflammatory cytokines by immune cells, thereby compromising the host’s defense response [33].
de Souza Costa et al. [34] demonstrated that there was no alteration in the pulp tissue when a light-cured RMGIC was used as a pulp capping agent in very deep cavities in non-conditioned dentin (i.e., RDT up to 0.3 mm). On the other hand, the application of a restorative RMGIC to dentin that had been conditioned with polyacrylic acid led to significant and permanent alterations in the human dentin-pulp complex [34].
The dental materials and their constituent parts undergo initial in vitro testing for mutagenesis and cytotoxicity. Understanding the protective properties of the dentin-pulp complex is crucial for optimizing material usage and formulating indications to prevent serious pulp damage. In order to maintain vitality, the dentin-pulp complex can adapt to a variety of stimuli and trigger a defense response. The substance will cause a biological reaction when it comes in contact with living tissues and its cytotoxicity may cause changes in metabolism up to cellular death. The first step in determining a material’s biocompatibility is to conduct cytotoxicity tests [35].
In vitro studies conducted by Leyhausen et al. [23], Rodriguez et al. [24], Mohd Zainal Abidin et al. [25], Koohpeima et al. [26] and de Souza Costa et al. [27] evaluated the cytotoxicity of conventional GIC and RMGIC using experimental cells derived from human i.e., HGF, SHED, HDPF, and MDPC-23. The results of these studies commonly stated that the cytotoxic effects of conventional GIC were significantly lesser than RMGIC. In another study included in this systematic review conducted by Sun et al. [28], the researchers examined the effects of 6 different contemporary dental restorative materials on human primary cells in vitro. The findings showed that Fuji II and Fuji II LC were not harmful to human pulp cells, while Vitremer exhibited significant cytotoxicity, likely attributed to its high content of TEGDMA. It was also concluded that cytotoxicity was dose-dependent [28].
Eskandarizadeh et al. [21], Ribeiro et al. [20] and Mousavinasab et al. [22] conducted controlled clinical trials by creating deep cavities prepared in human premolars. They lined the cavities using conventional GIC and RMGIC and then restored them with composite resin. After time intervals of 5, 7, or 30 days, the teeth were extracted, and processed for histological evaluation of the pulp, presence of microorganisms and the RDT between the cavity floor and the pulp was measured. They provided insight into important elements related to the use of RMGIC as a liner in deep cavities, such as the inflammatory response, the presence of bacteria, the formation of tertiary dentin, odontoblastic alterations, and tissue disorganization. Notably, the results of these clinical trials show that RMGICs typically cause a higher early-stage inflammation than conventional GICs, even though both cement types cause some inflammation after 5 and 7 days. Nonetheless, the overall rate of severe inflammation for both groups remains low, suggesting an acceptable level of biocompatibility. The authors attributed the low-level presence of microorganisms to insufficient isolation or contamination during the procedure.
Tertiary dentin formation after the pulp capping procedure is often thought of as a crucial long-term parameter determining clinical success. In the above-mentioned studies, there were no significant differences between the amount of tertiary dentin formed by conventional GIC and RMGIC. However, one must keep in mind that the amount of tertiary dentin formed is not always indicative of a successful clinical pulpal response. Additional factors, such as tissue disorganization and odontoblastic alterations, also contribute to an overall satisfactory level of biocompatibility of both test materials.
CONCLUSIONS
In the current systematic review, 7 out of 9 included studies presented low risk of bias whereas 2 studies showed moderate risk of bias. The evidence in this review suggests favorable results with conventional GIC as compared to RMGIC in terms of biological response of pulp when used in vital pulp therapies. Further long-term clinical trials are needed to effectively establish the conclusion.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Singh S, Kulkarni G, Mohan Kumar RS, Jain R.
Data curation: Singh S, Kulkarni G, Jain R.
Formal analysis: Jain R, Lokhande AM, Sitlaney TK, Ansari MHF.
Investigation: Singh S, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF.
Methodology: Singh S, Kulkarni G, Mohan Kumar RS, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF.
Project administration: Singh S, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Software: Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Supervision: Singh S, Kulkarni G, Mohan Kumar RS, Jain R.
Validation: Singh S, Kulkarni G, Mohan Kumar RS, Agarwal NS.
Visualization: Singh S, Kulkarni G, Mohan Kumar RS, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Writing - original draft: Singh S, Kulkarni G, Mohan Kumar RS, Jain R, Lokhande AM, Sitlaney TK, Ansari MHF, Agarwal NS.
Writing - review & editing: Singh S, Kulkarni G, Mohan Kumar RS, Lokhande AM, Sitlaney TK.