Can different agents reduce the damage caused by bleaching gel to pulp tissue? A systematic review of basic research
Article information
Abstract
Objectives
This study aimed to investigate the effectiveness of different topical/systemic agents in reducing the damage caused by bleaching gel to pulp tissue or cells.
Materials and Methods
Electronic searches were performed in July 2023. In vivo and in vitro studies evaluating the effects of different topical or systemic agents on pulp inflammation or cytotoxicity after exposure to bleaching agents were included. The risk of bias was assessed.
Results
Out of 1,112 articles, 27 were included. Nine animal studies evaluated remineralizing/anti-inflammatories agents in rat molars subjected to bleaching with 35%–38% hydrogen peroxide (HP). Five of these studies demonstrated a significant reduction in inflammation caused by HP when combined with bioglass or MI Paste Plus (GC America), or following KF-desensitizing or Otosporin treatment (n = 3). However, orally administered drugs did not reduce pulp inflammation (n = 4). Cytotoxicity (n = 17) was primarily assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay on human dental pulp cells and mouse dental papilla Cell-23 cells. Certain substances, including sodium ascorbate, butein, manganese chloride, and peroxidase, were found to reduce cytotoxicity, particularly when applied prior to bleaching. The risk of bias was high in animal studies and low in laboratory studies.
Conclusions
Few in vivo studies have evaluated agents to reduce the damage caused by bleaching gel to pulp tissue. Within the limitations of these studies, it was found that topical agents were effective in reducing pulp inflammation in animals and cytotoxicity. Further analyses with human pulp are required to substantiate these findings.
Trial Registration
PROSPERO Identifier: CRD42022337192
INTRODUCTION
Patient dissatisfaction with tooth staining has led to a surge in demand for dental bleaching procedures [12]. These procedures, which can be performed either in-office or at home, utilize varying concentrations of peroxides to achieve aesthetically pleasing teeth [3]. However, prior research has highlighted potential harmful effects associated with these procedures. These include an increase in pulp inflammation in both humans and animals, a decrease in the mineralization potential of pulp cells, and severe postoperative dental sensitivity [1456789]. Furthermore, patients who undergo in-office bleaching have reported a higher incidence and intensity of tooth sensitivity compared to those who opt for at-home bleaching. This is believed to be due to the use of high concentrations of hydrogen peroxide (HP) [10].
The active ingredient in bleaching gels is typically HP or one of its precursors, such as carbamide peroxide (CP). The process of tooth whitening involves an oxidation-reduction reaction, which is initiated by reactive oxygen species (ROS) of low molecular weight. These ROS are released by peroxide-containing bleaching gels and are able to penetrate hard tissues [111213]. However, HP can induce structural and mechanical changes in enamel, including increased roughness, and decreased flexural strength and hardness [141516]. Furthermore, the ROS produced by HP diffusion can infiltrate the pulp tissue, potentially leading to cell membrane damage, apoptosis, enzymatic deregulation, and an increase in the immunolabeling of pro-inflammatory markers such as substance P (SP), tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-17 [61718192021]. Research has indicated that the intensity of the inflammatory response is influenced by the concentration of the bleaching gel, the number of bleaching sessions, and the thickness of the enamel and dentin layers [517]. Moreover, the pH and chemical composition of the bleaching agent can also affect these outcomes [12223].
Various agents and protocols have been explored to mitigate the damage caused by HP to dental tissues. These include the use of different topical and systemic anti-inflammatory or antioxidant drugs, as well as remineralizing agents [132425]. For example, Otosporin and other topical agents have demonstrated some anti-inflammatory potential in pulp tissue following dental bleaching in animal studies [31225]. However, this potential has not been observed with oral anti-inflammatories [325]. Protocols that involve the use of remineralizing agents to minimize the adverse effects of bleaching agents have also been investigated. One study noted reduced HP penetration and pulp damage after using a mixture of MI Paste Plus (GC America, Alsip, IL, USA) with the bleaching gel, while another reported a decrease in the inflammatory reaction after using a bioactive glass-ceramic-based gel during the bleaching process [112].
Despite the numerous protocols and substances that have been tested, their effectiveness remains a topic of debate. For instance, while substances such as fluoride have demonstrated an increase in enamel hardness, they have not shown any significant effect on cell metabolism and mineralization in vitro [6]. Likewise, certain topical or oral agents have not shown any significant impact on the response of pulp tissue in bleached teeth [1726]. Given the broad spectrum of agents that have been assessed to mitigate the adverse effects on dental pulp and the cytotoxicity of bleaching gels, the aim of this systematic review is to examine the efficacy of various topical or systemic agents in reducing damage to pulp tissue or pulp cells caused by bleaching.
MATERIALS AND METHODS
Protocol
The present systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [27]. A research protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) database, under the identifier CRD42022337192.
Eligibility criteria
The inclusion criteria were as follows: 1) laboratory or animal studies that evaluated the effects of various topical or systemic agents in minimizing the damage inflicted on pulp tissue during dental bleaching with HP and/or CP gels, and 2) in vitro studies that investigated the efficacy of agents in reducing cytotoxicity in pulp cells caused by HP and/or CP. The exclusion criteria included: 1) studies that assessed a bleaching gel other than HP and/or CP, 2) studies that lacked a bleached control group for comparison, 3) studies that evaluated light therapy, and 4) studies for which the full text was not available. No restrictions were imposed on the language and date of publication.
The population, intervention, comparison, outcome (PICO) approach was used to address the following question: “Can various topical or systemic agents reduce the damage inflicted on pulp tissue or pulp cells by bleaching gel (HP and/or CP)?”. The study population (P) consisted of pulp tissue or pulp cells. The intervention (I) involved bleaching with HP and/or CP gel, supplemented with additional agents. The comparison (C) was made with pulp tissue or cells exposed to bleaching gels without the use of supplementary agents. The primary outcome (O) was the inflammatory response in pulp tissue for in vivo studies, and cytotoxicity for in vitro studies. Secondary outcomes included the formation of hard tissue in vivo and oxidative stress in vitro.
Search strategy
Electronic searches were conducted in the PubMed/Medline, Scopus, Embase, Cochrane Library, and Web of Science databases up to July 2023. The gray literature was also reviewed via Google Scholar, where the first 300 results, sorted by relevance, were evaluated [28]. Manual searches were additionally conducted within the reference lists of the selected articles. The search strategy employed a mix of keywords and Medical Subject Heading (MeSH) terms, combined with the Boolean operators “AND” and “OR,” as detailed in Supplementary Table 1.
Study selection
Two independent authors (Batista LAS and Arantes LC) conducted the study selection in a two-step process. In the first step, the authors evaluated the titles and abstracts of the studies obtained from the searches. Duplicate entries were eliminated using reference manager software (EndNote; Thomson Reuters, Toronto, Canada). Studies whose titles and abstracts met the eligibility criteria were immediately included. If the titles and abstracts of studies provided insufficient information for a decision, their full texts were downloaded for further review. In the second step, a full-text assessment of the remaining records was conducted. Studies that met the eligibility criteria upon full-text review were also included. Any disagreements were resolved through discussion, and if necessary, a third author (Benetti F) was consulted. The Cohen’s kappa coefficient was calculated to measure the inter-investigator agreement during the study selection process [29].
Data extraction and analyses
Two authors (Batista LAS and Chaves HGS) collected the following data from the included studies: the first author’s surname, year of publication, experimental model, groups, bleaching gel protocol, additional protocol, and period of analysis. They also tabulated the data of the analyses, which served as a method for assessing the outcomes and main results. A pre-tested data extraction form in an Excel spreadsheet was utilized for this data collection. Following this, a third author (Reis-Prado AH) reviewed the collected data.
Synthesis of results
The selected studies were examined for methodological homogeneity to determine whether a quantitative analysis could be applied to these studies. However, due to significant heterogeneity in the evaluated concentrations of bleaching gels, the use of topical or systemic agents, cell types, assessment methods, and observation periods, a meta-analysis was not conducted. Instead, a qualitative synthesis of the results from the included studies was provided.
Risk of bias assessment
Two investigators (Batista LAS and Arantes LC) independently evaluated the risk of bias in the selected in vivo studies. This evaluation was conducted in accordance with a modified version of the “Systematic Review Centre for Laboratory Animal Experimentation” (SYRCLE’s RoB tool) 10-criteria tool [30]. The criteria included: adequate generation of allocation sequence, similarity of groups at baseline or adjustment for confounders due to group differences, adequate allocation concealment, random housing, blinded intervention/outcome assessment, random sample selection for outcome assessment, completeness of outcome data, selective outcome reporting, presence of other biases, and justification of sample size to enhance the characterization of reporting in the animal studies. A “no” judgment indicated a high risk of bias, “yes” signified a low risk of bias, and “unclear” denoted either a lack of information or uncertainty regarding bias.
For in vitro studies, the Joanna Briggs Institute (JBI)’s Critical Evaluation for Experimental Studies was employed, with some modifications [731]. The checklist included the following items: a clearly stated aim, justification for the sample size, sample randomization, blinding of outcome assessors regarding treatment allocation, equivalence of control and intervention groups at baseline, identical treatment of control and intervention groups except for the interventions being compared, a clear description of the treatment protocol, standardization of outcome assessment, a reliable method for measuring the outcome, and an adequate statistical approach. Each item on the JBI scale was scored as follows: 0 for items not reported or inadequately reported, indicating a high or unclear risk of bias; 1 for items reported and adequately addressed, indicating a low risk of bias. Any doubts or discrepancies were discussed until a consensus was reached. If a resolution could not be achieved, a third examiner (Reis-Prado AH) was consulted.
RESULTS
Study selection
The flowchart of the search process is displayed in Figure 1. In total, 1,112 records were screened. After the initial screening (Step 1), 43 studies were selected for full-text assessment (Step 2). Then, 16 studies were excluded, and the reasons for their exclusion can be found in Figure 1 and Supplementary Data 1 [32333435363738394041424344]. Twenty-seven studies meeting the inclusion criteria were selected for qualitative analysis [13611121723242526454647484950515253545556575859606162].
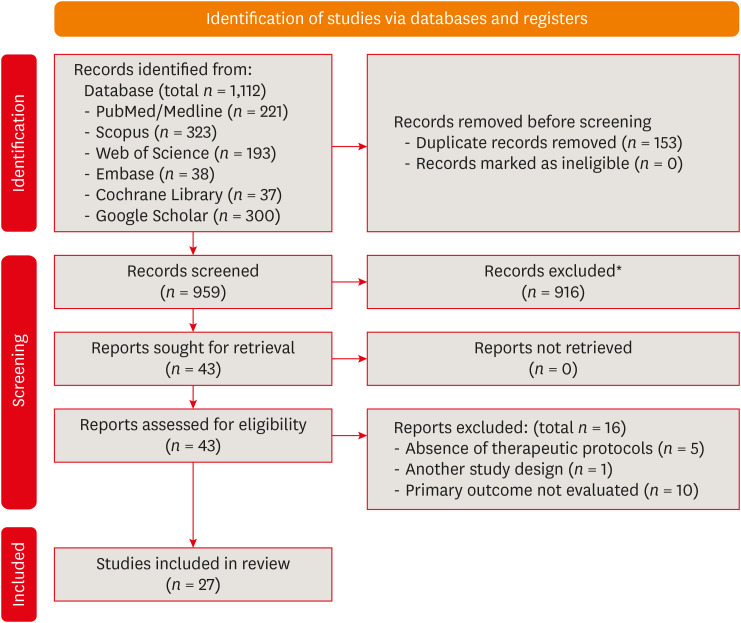
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
*Exclusion of records was performed by authors of this systematic review.
The inter-examiner kappa scores, which indicate agreement between reviewers, were found to be 0.969 for references retrieved from PubMed/Medline, 1.000 for Scopus, 0.818 for Embase, 1.000 for the Cochrane Library, 1.000 for Web of Science, and 1.000 for references in Google Scholar. These values, according to the Landis & Koch scale, suggest an almost perfect level of agreement among reviewers during the study selection process [29]. No further records were discovered through manual searches in the reference lists.
Characteristics of the included studies
Table 1 summarizes the 9 in vivo studies that evaluated the effects of different interventions on dental pulp during the bleaching process [1311121725265860]. The primary experimental model used was rat teeth, specifically the upper molars, as in 8 studies. The studies utilized HP gels at concentrations of 35% [1311121726], 37.5% [60], or 38% [25]. The application times for the bleaching gels varied, with the majority of studies adhering to a 30-minute protocol [1111226]. In terms of supplementary protocols, certain substances were combined with the bleaching gel, including MI Paste Plus remineralizer and Biosilicate bioglass [112]. Other substances were applied topically either before or after the application of the bleaching gel. These included desensitizing potassium nitrate and sodium fluoride (Des KF), Otosporin anti-inflammatory, curcumin gel, carvedilol gel, and again, MI Paste Plus remineralizer, and Biosilicate bioglass [131112252658]. Studies also evaluated the effects of oral anti-inflammatory and analgesic agents such as ibuprofen and Tylenol, as well as ascorbic acid [317255860]. The analysis periods ranged from 0 to 30 days post-dental bleaching, with a 2-day period being the most commonly reported (in 7 studies).
Table 2 presents data from 19 in vitro studies that explored various agents aimed at reducing cytotoxicity during the bleaching process [6232445464748495051525354555657]. The majority of these studies utilized human dental pulp cells (HDPCs) and mouse dental papilla Cell-23 (MDPC-23) cells, with each cell type being used in 10 studies. Of these, 8 studies employed enamel/dentin discs in conjunction with pulp cells, while the remaining studies used extracts from bleaching gel [623454757596062]. The bleaching gel most frequently evaluated was the HP-based gel, which was used in varying concentrations and volumes, and applied over periods ranging from 15 minutes to 12 days [2324454648495051525354555657596062].

Main characteristics and effects of protocols on cell viability/cytotoxicity, morphology, and mineralization
A wide range of substances were used in supplementary protocols, including cyclosporine A, N-acetylcysteine, horseradish peroxidase, catalase, peroxidase, ferrous sulfate, manganese chloride, indole-3-acetic acid, cinnamaldehyde, alpha-tocopherol, fluoride, butein, proliferator-activated receptor gamma, sulfuretin, pachymic acid, low-molecular weight protamine, sappanchalcone, sodium ascorbate, and polymeric catalyst primer [623244546474849505152535455565758596062]. These substances were predominantly applied prior to the application of the bleaching gel [4748505152535562]. Analysis periods of 1, 6, and 24 hours were most frequently reported.
Tables 1, 2, and 3 provide a summary of the results for inflammatory reactions, cell viability/mineralization, and oxidative stress/protein expression, respectively. The main findings of these outcomes are described below.
Inflammatory response
Pulp inflammation was assessed using hematoxylin-eosin (HE) staining (Table 1). In 6 studies, a decrease in the inflammatory response was noted with some of the agents assessed, particularly at 24 and 48 hours after dental bleaching. For example, the topical application of Ostoporin following 35% HP, the use of Biosilicate bioglass for 20 minutes prior to or in combination with 35% HP gel, the mixture of MI Paste Plus with 35% HP, and the topical application of 2% Des KF for 10 minutes, 30 minutes prior to 38% HP, all demonstrated a significant decrease in the pulp inflammation induced by the bleaching gel [1311122558]. On the other hand, the application of 2.5% Carvedilol gel for 10 minutes, or curcumin gel, following 35% HP, did not exhibit any impact on the inflammatory response [2658]. Likewise, the administration of ascorbic acid or Tylenol also did not significantly affect pulp inflammation. A few articles presented conflicting results concerning the effects of various formulations of ibuprofen applied before and/or after HP [255860].
Four studies examined the immunolabeling of pro-inflammatory markers, while one study evaluated an anti-inflammatory marker, IL-10 [3112560]. The previous application of Des KF reduced the levels of SP and calcitonin gene-related peptide at 24 and 48 hours [25]. Furthermore, the application of Otosporin following bleaching, and the oral administration of Tylenol prior to bleaching, resulted in a decrease in SP immunolabeling both immediately and at 24 hours in the pulp tissue [3]. The use of Otosporin also reduced the presence of TNF-α in pulp tissue, but it did not influence IL-6 and IL-17 immunolabeling. The application of an ibuprofen-loaded hydrogel and/or nanogel led to a decrease in IL-1β immunolabeling at 24 hours and an increase in IL-10 immunolabeling at 14 days, respectively.
Hard tissue formation
Hard tissue formation following bleaching was evaluated in 3 studies utilizing HE [11226]. The use of 2.5% carvedilol gel for 10 minutes post-dental bleaching did not impact the formation of hard tissue [26]. Conversely, protocols that incorporated remineralizing agents like Biosilicate bioglass and MI Paste Plus in combination with HP gel significantly diminished the development of tertiary dentin after 30 days [112].
Cytotoxicity
Cytotoxicity was primarily evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in 17 studies, as shown in Table 2. Many of these studies found that additional protocols helped to mitigate the cytotoxicity caused by bleaching agents [2446474849505253545556596062]. Various substances and reagents, including 10% sodium ascorbate, butein, 10 mM of vitamin E alpha-tocopherol, indole-3-acetic acid, 1-10 mg manganese chloride, peroxidase, catalase, and horseradish peroxidase, were found to be effective in reducing cytotoxicity [2324475354575960]. This was particularly the case when these substances were applied prior to exposure to the bleaching agent.
The results were inconsistent when using a topical application of ferrous sulfate mixed with 35% HP for 3 15-minute sessions. One article reported a reduction in cytotoxicity with this substance, while another study demonstrated the opposite effect [2345]. Furthermore, a 1-minute fluoride application following 16% CP did not appear to impact the cytotoxicity in MDPC-23 cells [6].
Oxidative stress evaluation
Oxidative stress (Table 3) was primarily evaluated using carboxy-H2DCFDA fluorescence and through the expression of heme oxygenase (HO)-1 in 5 studies each. In 3 in vitro studies, the expression of HO-1 and/or Nrf2 was assessed using Western blot, immunofluorescence, and densitometric analysis [24505455]. Two studies demonstrated an increase in HO-1 and Nrf2 expressions following a protocol that involved 20 µM cinnamaldehyde prior to 300 µM of HP, and indole-3-acetic acid, with concentrations ranging from 1 to 300 μM, after exposing cells to HP for 24 hours, respectively [5455]. Additionally, 6 studies evaluated ROS through fluorescence and flow cytometry [244649505455]. Some of these studies reported a significant reduction in ROS production in HDPCs when using 5–40 μM sappanchalcone, 6–10 mg/mL manganese chloride, 100 MOI of proliferator-activated receptor gamma, and 2.5–20 μM butein prior to exposure to 1 mM and 150 μM HP [2446495960].
Critical appraisal of the studies
Risk-of-bias analyses are presented in Supplementary Tables 2 and 3, Figures 2 and 3. Supplementary Table 2 and Figure 2 summarize the risk of bias in in vivo studies, utilizing the SYRCLE tool. Generally, the reports were adequate in terms of the similarity of groups at baseline, blinded outcome assessment, incomplete outcome data, and other sources of bias, with only 1 study having this domain scored as unclear [25]. However, most evaluated items lacked the necessary information or were reported inappropriately for a proper judgment.
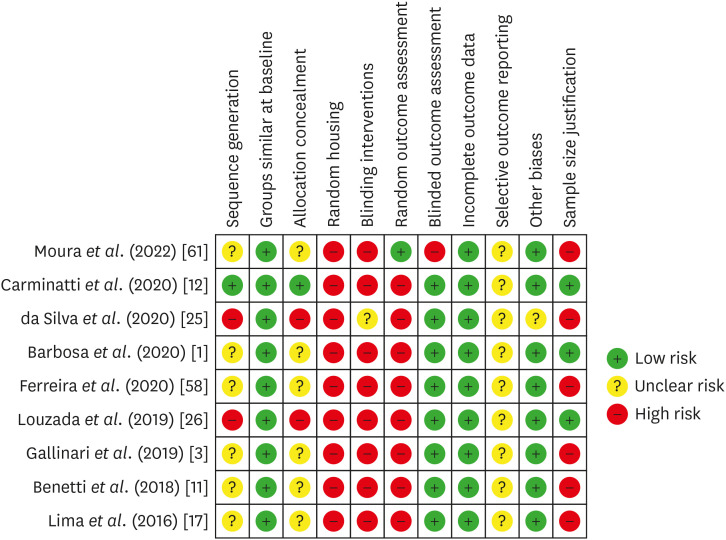
Risk of bias in individual animal studies according to the SYRCLE’s RoB tool for assessing risk of bias.
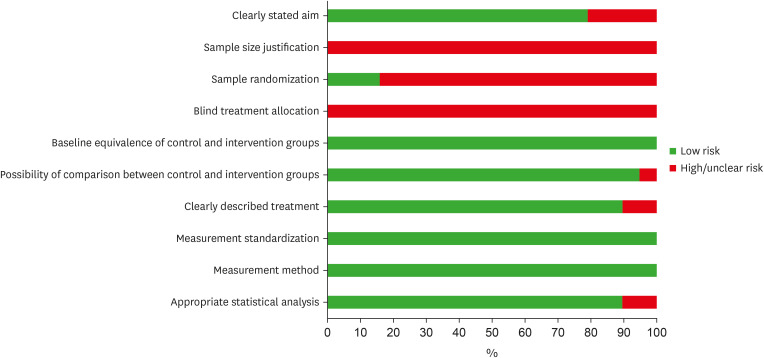
Risk of bias assessment of the eligible in vitro studies, by the percentage of the scores attributed to each evaluated study (Joanna Briggs Institute’s Critical Appraisal Checklist).
The JBI tool was utilized to critically appraise in vitro studies (Supplementary Table 3, Figure 3). None of the studies included in the review provided justification for their sample size or reported any blinding. Furthermore, a high risk of bias was identified when a random sample was present. All studies demonstrated equivalence between control and intervention groups at baseline, standardized measurement, and a reliable method of analysis. Most of the selected studies exhibited a low risk of bias in the remaining items.
DISCUSSION
The present systematic review investigated the effectiveness of various topical or systemic agents in reducing pulp inflammation following dental bleaching, as well as their ability to decrease the cytotoxicity induced by bleaching gel in pulp cells. The review also considered in vivo hard tissue formation and in vitro oxidative stress. A total of 24 studies were included, with several protocols being evaluated. Despite noticeable methodological differences among the selected studies, the majority of the anti-inflammatory, analgesic, and remineralizing agents tested showed a significant decrease in pulp inflammation in animal models and a reduction in cytotoxicity following exposure to bleaching agents. However, no in vivo studies were found that evaluated topical or systemic agents in the pulp tissue of bleached human teeth.
Bleaching gels containing peroxide have been shown to potentially cause severe inflammation and necrosis in pulp tissue, particularly when used in high concentrations [456364]. It is well-established that dental bleaching releases a significant amount of SP, a neurotransmitter that modulates pain [365]. In a clinical setting, this inflammatory process often manifests as intense tooth sensitivity following a bleaching procedure [8966]. Initially, the pulp responds to mild stimuli by depositing reactionary dentin through the odontoblast cell layer [735]. However, more intense stimuli can lead to increased inflammation and the death of odontoblasts. Consequently, differentiated dental pulp stem cells and other resident cells, such as fibroblasts, are tasked with producing reparative dentin [20216768].
Regarding the inflammatory process, animal studies have demonstrated a significant reduction in pulp inflammation and the immunolabeling of pro-inflammatory markers following the topical application of Otosporin after the use of 35% HP gel [31158]. Otosporin, a drug known for its high penetration capacity, possesses anti-inflammatory and immunosuppressant properties and contains neomycin sulfate, polymyxin B sulfate, and hydrocortisone [69]. Prior research has suggested that the corticosteroid action of Otosporin can decrease vascular permeability, thereby reducing the inflammatory response [6970]. Additionally, these studies propose that microcracks in the surface area of dental enamel could enhance the penetration of topical agents, especially after bleaching with HP, which increases enamel porosity [71]. A recent clinical study evaluating the topical application of Otosporin prior to in-office bleaching found that this drug did not significantly reduce the risk or intensity of tooth sensitivity [8]. However, the studies included in this systematic review specifically assessed the use of Otosporin only after bleaching, indicating a need for further clinical evaluation. Conversely, other topical anti-inflammatory or antioxidant agents, such as curcumin gel or carvedilol gel, did not significantly reduce pulp inflammation when applied after bleaching [2658], underscoring the properties of Otosporin. In terms of the in vivo methods used to assess pulp inflammation, all the selected animal studies utilized HE staining and/or immunolabeling of inflammatory markers, along with a scoring system for outcome evaluation. It is important to note, however, that this approach may have methodological limitations due to its subjective nature. To mitigate these limitations, it is suggested to consider alternative and more precise measurements for each sample, such as cell counting or the use of molecular biology approaches.
The high diffusion of HP and the indiscriminate action of free radicals can modify the morphology of hard tissues, thereby weakening the tooth structure and promoting toxic effects on pulp cells [14772]. Consequently, protocols that incorporate desensitizing and remineralizing agents either before, after, or in combination with the bleaching gel have been evaluated both in vivo and in vitro [161245]. The use of bioactive glass-ceramic (Biosilicate) either before or in combination with 35% HP gel, MI Paste Plus (a paste made up of casein phosphopeptide and amorphous calcium phosphate) combined with 35% HP, and 2% Des KF have demonstrated positive effects in mitigating pulp inflammation induced by HP [11225]. Moreover, a prior in vitro study revealed that the desensitizing agents 2% Des KF and MI Paste Plus can decrease the HP penetration into the pulp chamber without undermining the effectiveness of the bleaching procedure [44].
These remineralizing agents help maintain an alkaline environment, thereby reducing the acidification caused by HP. They also release fluoride, calcium, and phosphate ions, which enhance the mineralized structures of bleached teeth [124473]. As a result, both Biosilicate and MI Paste Plus have been found to reduce the formation of tertiary dentin at 30 days [112]. This suggests a decrease in the aging of pulp tissue and potential impairment of the dental pulp's defense mechanisms [19]. The results concerning MI Paste Plus are consistent with a previous clinical study. This study demonstrated that the application of casein phosphopeptide and amorphous calcium phosphate paste during at-home tooth bleaching with 20% CP can reduce tooth sensitivity while preserving the effect of color change [74]. Regarding Biosilicate, one study found it to be effective in increasing enamel microhardness following tooth bleaching [75]. However, there is still a dearth of clinical studies evaluating postoperative sensitivity.
The oral use of ibuprofen was found to be unsatisfactory in reducing the inflammatory response in vivo [2558]. This aligns with a clinical study that assessed the impact of perioperative ibuprofen on tooth sensitivity resulting from in-office bleaching. The study found that while ibuprofen did lessen the intensity of tooth sensitivity, it was unable to prevent it entirely, with its effects lasting only up to an hour post-bleaching [66]. Another clinical study demonstrated that ibuprofen could decrease tooth sensitivity during the treatment period, but not beyond its completion [76]. However, a recent study presented promising results in reducing pulp inflammation when ibuprofen-loaded hydrogel and/or nanogel were incorporated into the bleaching gel [60]. These conflicting results may be attributed to the carriers used in the latter study, which could regulate drug release and enhance the potential for topical penetration compared to traditional oral drug delivery. Therefore, further studies that take into account these varying drug delivery systems are necessary to ascertain the efficacy of ibuprofen in mitigating the damage caused by bleaching.
All eligible in vivo studies utilized rat teeth to evaluate the effectiveness of various agents in minimizing pulp damage following bleaching. This selection may be due to ethical considerations, the opportunity for specimen standardization, and a tissue response that closely mirrors that of a human tooth. It is worth noting that the majority of studies using rat molars, identified in this systematic review, adhered to a standardized protocol for rat bleaching from a previous study [63].
Rat molars are considered to closely resemble human teeth in terms of enamel and dentin proportions, as well as pulp tissue response [1163]. However, rat incisors, which continuously grow and lack enamel on the buccal surface, may elicit a different dental pulp response from the exterior [63]. Despite this, there is a clear need for an animal model to assess the impact of various agents on pulp tissue, as no in vivo studies identified in this systematic review have been conducted on human teeth. This is likely due to the challenges associated with obtaining extracted teeth suitable for analysis. While the anatomical and histological characteristics of rat molars do mirror those of human teeth, these findings cannot be directly applied to humans. Therefore, further research is necessary to comprehend the outcomes of using these agents in humans.
For the in vitro assessment of dental pulp cells, 10 studies used HDPCs to evaluate the cell damage provoked by HP and/or CP [24294546485051545556]. This particular cell line may more accurately mimic the potential effects of various materials and dental therapies evaluated in vitro [77]. Moreover, while the majority of in vitro studies employed the MTT assay to ascertain cell viability, another significant evaluation parameter was the production of ROS, which is directly linked to the initiation of cell damage [77]. Bearing this outcome in mind, most protocols mitigated the cytotoxicity of bleaching agents. These agents consisted of organic compounds derived from fungi and plants, such as various antioxidants (butein, sodium ascorbate), and enzymes (peroxidase, catalase, horseradish peroxidase), along with other reagents. These compounds enhanced cell viability and diminished oxidative stress, particularly when applied prior to the bleaching agent. However, only 5 studies incorporated enamel/dentin discs during cell viability assays [623454757].
Using enamel/dentin discs for in vitro models is crucial for creating a model that closely resembles the clinical condition, where the whitening gel is applied directly to the enamel. This method facilitates the evaluation of peroxide diffusion and its impact on pulp cells. A correlation between the quantity of HP that reaches the pulp chamber and the thickness of the specimen has been previously established [78]. Consequently, in this systematic review, the studies that utilized enamel/dentin discs were categorized based on the discs’ diameter and thickness. A standardized thickness of 2.3 mm has been recently adopted in studies conducted by Dr. de Souza Costa’s research group [57596062], which simulates a critical distance for HP diffusion to reach the pulp chamber. Conversely, when the enamel/dentin disc was not employed, small quantities (typically in µM) or percentage HP (such as 0.018%) were applied. In general, high concentrations of HP and extended application durations can result in rapid diffusion of the bleaching agent through the dentinal tubules [4579].
Additionally, HP activates proteolytic enzymes in dentin, which in turn accelerates the degradation of the extracellular matrix [4580]. However, the thickness of the discs used in these studies can impact the diffusion of the bleaching agent through the dentinal tubules, thereby influencing the metabolic activity of the pulp cells. Therefore, it is crucial to establish standardized in vitro models and additional protocols to assess the transdentinal diffusion capacity of ROS released from bleaching gels. This will also help determine the appropriate concentration of substances capable of minimizing cell damage. However, it is important to note that most of the agents used in in vitro studies have not yet been evaluated in vivo or in clinical trials. Are these agents viable alternatives? For instance, only 1 clinical study has used ascorbic acid following the application of the bleaching gel, with the intention of immediate tooth restoration [81]. This systematic review identified only 1 in vivo study that evaluated ascorbic acid. In this study, the acid was orally administered to animals and was unable to significantly reverse the damage caused by HP in the pulp tissue [17]. Another consideration is that while some studies used enamel/dentin discs [23344547575962], none investigated the effects of the bleaching gel or the different agents in a 3D cell culture. This is a crucial step to make the model more clinically relevant. However, while some agents examined in the included in vitro studies have demonstrated a reduction in cell damage caused by bleaching gels, most of the tested agents are still far from being clinically applicable. Therefore, reducing the concentration of the bleaching gel could be a more clinically accessible and easily reproducible alternative to minimize the damage caused by bleaching procedures using highly concentrated gels [3438].
Although basic research provides insight into tissue response and cell behavior during dental procedures, it is crucial to conduct clinical evaluations of the various additional protocols discussed here. In the in vivo studies, the critical appraisal was assessed using the SYRCLE’s RoB tool, which identified an unclear-to-high risk of bias in most domains. However, a satisfactory report was noted for the presence of groups that were similar at baseline, blinding of outcome assessment, incomplete outcome data, and other sources of bias. In contrast, for in vitro studies, a low risk of bias was predominantly observed using a modified JBI tool. Despite this, some methodological issues were detected, such as the lack of justification for sample size, absence of blinding to treatment assignment, and lack of random sampling.
Moreover, it should be noted that another limitation of this systematic review is that it primarily investigated protocols involving high-concentration peroxide gels and short-term analysis. However, the European Union Council Directive 2011/84/EU (which amends EU Council Directive 76/768/EEC) recommends the use of hydrogen peroxide at low concentrations, up to 6% HP, in a clinical setting due to the increased damage caused by high concentrations of HP [82]. Despite the variety of agents analyzed in this systematic review, all studies indicated significant damage to the pulp tissue, underscoring the need for caution. None of the agents were found to be completely effective in preventing damage to cells or pulp tissue from the bleaching gel. Therefore, it is recommended that future studies assess protocols that combine low-concentration bleaching agents with longitudinal analysis.
CONCLUSIONS
Few in vivo studies have been conducted to assess the efficacy of topical or systemic agents in mitigating the damage caused by bleaching gel on pulp tissue. Within the constraints of this study, it was determined that topical agents were successful in decreasing inflammation in the pulp tissue of animals. Moreover, the majority of topical agents evaluated in vitro demonstrated a reduction in cytotoxicity. However, further analysis using standardized protocols on human pulp is required.
Notes
Funding: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES, 88887.649870/2021-00; 88887.712700/2022-00) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 128044/2022-5).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Morgan LFSA, André CB, Suzuki TY, Benetti F.
Data curation: Batista LAS, Chaves HGS, Reis-Prado AH, Arantes LC, Benetti F.
Methodology: Reis-Prado AH, Chaves HGS, André CB, Suzuki TY, Benetti F.
Project administration: Benetti F, Reis-Prado AH, Morgan LFSA.
Resources: Morgan LFSA, Benetti F.
Supervision: André CB, Suzuki TY, Benetti F.
Validation: Batista LAS, Reis-Prado AH, Arantes LC.
Visualization: Batista LAS, Chaves HGS, Arantes LC.
Writing - original draft: Batista LAS, Reis-Prado AH, Chaves HGS, Arantes LC.
Writing - review & editing: Morgan LFSA, André CB, Suzuki TY, Benetti F.
References
SUPPLEMENTARY MATERIALS
Supplementary Data 1
List of excluded references (full text) sorted by reasons.
Supplementary Table 1
Search strategies used for the electronic databases
Supplementary Table 2
SYRCLE’s risk of bias tool for animal studies
Supplementary Table 3
Critical appraisal of included in vitro studies





