Physicochemical properties of a calcium aluminate cement containing nanoparticles of zinc oxide
Article information
Abstract
Objectives
This study evaluated the effect of different nanoparticulated zinc oxide (nano-ZnO) and conventional-ZnO ratios on the physicochemical properties of calcium aluminate cement (CAC).
Materials and Methods
The conventional-ZnO and nano-ZnO were added to the cement powder in the following proportions: G1 (20% conventional-ZnO), G2 (15% conventional-ZnO + 5% nano-ZnO), G3 (12% conventional-ZnO + 3% nano-ZnO) and G4 (10% conventional-ZnO + 5% nano-ZnO). The radiopacity (Rad), setting time (Set), dimensional change (Dc), solubility (Sol), compressive strength (Cst), and pH were evaluated. The nano-ZnO and CAC containing conventional-ZnO were also assessed using scanning electron microscopy, transmission electron microscopy, and energy-dispersive X-ray spectroscopy. Radiopacity data were analyzed by the 1-way analysis of variance (ANOVA) and Bonferroni tests (p < 0.05). The data of the other properties were analyzed by the ANOVA, Tukey, and Fisher tests (p < 0.05).
Results
The nano-ZnO and CAC containing conventional-ZnO powders presented particles with few impurities and nanometric and micrometric sizes, respectively. G1 had the highest Rad mean value (p < 0.05). When compared to G1, groups containing nano-ZnO had a significant reduction in the Set (p < 0.05) and lower values of Dc at 24 hours (p < 0.05). The Cst was higher for G4, with a significant difference for the other groups (p < 0.05). The Sol did not present significant differences among groups (p > 0.05).
Conclusions
The addition of nano-ZnO to CAC improved its dimensional change, setting time, and compressive strength, which may be promising for the clinical performance of this cement.
INTRODUCTION
Tricalcium silicate-based cements have been widely used in dentistry due to their excellent biological and physicochemical properties [1]. The precursor of this type of repairing cement was the Mineral Trioxide Aggregate (MTA), developed in the early 1990s [1]. Despite the clinical advantages of MTA in relation to other materials of similar application, this cement has several negative features, such as the longer setting time, the poor handling characteristic, low flow ability, high incidence of dental structure staining, solubility and disintegration higher than the limit proposed by the specification No. 57 of ANSI/ADA [12345]. In addition, Garcia et al. [6] have reported the presence and release of some heavy metals, such as arsenic, lead, and chromium by the Angelus MTA.
In order to overcome the limitations of MTA, new biomaterials, such as calcium aluminate cements (CACs), have been proposed and constantly evaluated in terms of their physicochemical, mechanical, and biological properties [789]. EndoBinder (Binderware, São Carlos, SP, Brazil - Patent Number PI0704502-6) is a CAC, and it has clinical applications similar to MTA, especially when used for pulp-capping, root perforations repair, and apical surgery as a retrograde filling material [78910]. Studies demonstrated that EndoBinder has similar biocompatibility and cytocompatibility to MTA [910]. According to Garcia et al. [8], the mechanical resistance of EndoBinder is greater than MTA. It is a cement basically composed of (% by weight) Al2O3 (≥ 68.5), CaO (≤ 31.0), SiO2 (0.3–0.8), MgO (0.4–0.5), and Fe2O3 (< 0.3) [78910].
The radiopacity of cement is provided by a radiopacifier that must be added to it in minimal quantities to not affect its physicochemical and biological properties [10]. The bismuth oxide (Bi2O3) ensured a proper radiopacity to the earlier versions of MTA due to the high atomic number of bismuth (Z = 83) [10]. However, the addition of this compound (20% by weight) into the MTA formulation affected its hydration mechanism, compromising the mechanical resistance, biocompatibility, and bioactivity of the cement [891112].
For this reason, studies have been carried out to test alternative radiopacifiers to Bi2O3, with emphasis on zirconium oxide (ZrO), barium sulfate (BaSO4), titanium oxide (TiO2) and zinc oxide (ZnO) [1314]. Aguilar et al. [14] have reported that the addition of ZnO (20% by weight) to CAC provided adequate radiopacity to the cement, as recommended by ISO 6876 standard [15]. Furthermore, Oliveira et al. [7] demonstrated greater mechanical resistance and lower levels of porosity to ZnO-containing CAC [7]. As regards the pH and calcium ions release, CAC had a similar performance to MTA when using this radiopacifier [16].
The addition of a radiopacifier in the proportion of 20% of its volume by weight, especially into mineral aggregate-based cements, affects their microstructure, making them more porous and mechanically fragile [111213]. Studies have reported the adequate physicochemical properties of calcium silicate-based cements and root canal sealers after adding different proportions of nanoparticulated ZnO (nano-ZnO) to them [1718].
Nanoparticles have ultra-small dimensions (1 to 100 nm in size) and a large surface area-to-volume ratio [19]. According to Raura et al. [19], the addition of different types of nanoparticles to biomaterials used in dentistry has shown promising results, especially in endodontics, due to their antimicrobial potential. Nanoparticles are able to reduce biofilm formation and increase the remineralization of dental structures affected by endodontic microorganisms [19]. Nano-ZnO-modified titanium implants have greater antibacterial activity, osteogenic potential, and corrosion resistance [20]. Other studies have also reported the advantages of using nano-ZnO on the toxicity mechanism of cells [21] and the antimicrobial activity of intracanal medication against Enterococcus faecalis [21].
To the best of our knowledge, no study has evaluated the effect of adding a reduced content of nano-ZnO on the physicochemical properties of mineral aggregate-based cements, especially CAC. Therefore, the purpose of the present study was to evaluate the radiopacity, setting time, dimensional change, solubility, compressive strength, and pH of CAC containing different proportions of nano-ZnO and conventional-ZnO. The null hypothesis tested was that there would be no difference in the physicochemical properties of the cements, regardless of the proportion of nano- and conventional-ZnO.
MATERIALS AND METHODS
Preparation of samples
The zinc oxide (radiopacifying agent) (Lagos Chemical Industry, Arcos, MG, Brazil) in its different forms (nano- and conventional-ZnO) was added to the CAC (EndoBinder, Binderware). Initially, the nano-ZnO, conventional-ZnO, and CAC powders were individually weighed in a precision analytical balance (Mettler-Toledo Ind. E Com. Ltda., Model PE 160, Barueri, SP, Brazil), according to the different tested ratios (Table 1). The conventional-ZnO and nano-ZnO were then added to the CAC, totaling 1.0 g of powder, and the following experimental groups were created: G1 (20% conventional-ZnO), G2 (15% conventional-ZnO + 5% nano-ZnO), G3 (12% conventional-ZnO + 3% nano-ZnO) and G4 (10% conventional-ZnO + 5% nano-ZnO. The proportion used for the CAC handling was 1.0 g of powder to 0.21 mL of distilled water, according to the manufacturer’s recommendations.
The morphological and chemical characteristics of the nano-ZnO used in the preparation of the cements and the CAC containing conventional-ZnO were analyzed with scanning electron microscopy (SEM) (JEOL JSM 6390 LV, Akishima, Japan), transmission electron microscopy (TEM) (JEOL, JEM 2100, Tokyo, Japan) and energy-dispersive X-ray spectroscopy (EDS).
Sample size calculation
For the setting time test, the number of repetitions (n = 3) followed the ISO 6876 standard [15]. The number of repetitions (n = 6) for the dimensional change and the solubility tests followed specification No. 57 of ANSI/ADA [5], with modifications proposed and validated by Carvalho-Junior et al. [22]. The water in which the specimens were immersed during the dimensional change test was used to measure the pH, totaling 6 repetitions. For the radiopacity and compressive strength tests, the sample size was calculated with the software G*Power version 3.1.9.6 (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/). Based on the study by Guerreiro-Tanomaru et al. [17] (radiopacity) and Bernardi et al. [23] (compressive strength), the following parameters were adopted: α error = 0.05 (5% significance level), test power (1-β) = 0.80 and an effect size = 1. A total number of 6 specimens per experimental group was determined for each test.
Setting time
Plaster molds containing disc-shaped cavities (10 mm inner diameter × 1 mm in thickness) were fabricated and stored at 37°C and 95% relative humidity, following the ISO 6876 standard [15]. After 24 hours, the cavities were filled with the experimental cement (n = 3). A Gillmore-type needle (100 ± 0.5 g and a cylindrical tip 2.0 ± 0.1 mm in diameter) was perpendicularly applied to the surface of the cements, at alternate time intervals and in different areas, producing markings. The process was repeated until the markings were no longer visible. The setting time (minutes) was calculated from the beginning of the mixture until the moment when the Gillmore-type needle no longer produced markings on the surface of the cements.
Dimensional change
Twelve specimens for each experimental group were used, 6 for the 24 hours and 6 for the 30 days of analysis (n = 6). The specimens were fabricated in Teflon molds (3 mm inner diameter × 3.58 mm in thickness), according to Carvalho-Junior et al. [22] methodology.
Five minutes after the beginning of the cement mixture, the set was transferred to an oven at 37ºC ± 1ºC, with a relative humidity of 95%, for 24 hours. After, the cement surface was smoothed with abrasive sandpaper #600 (3M, São Paulo, SP, Brazil) under copious distilled water irrigation. The specimens of each group were removed from the molds and their lengths after setting (Linitial) were measured with a mechanical thickness gauge (Model 7360; Mitutoyo, Suzano, SP, Brazil). Next, the specimens were placed in individual containers containing 2.24 mL of distilled water and kept in an oven at 37ºC ± 1ºC, for 24 hours (G24h) or 30 days (G30d). At the end of the different experimental periods, the specimens were removed from the containers, and the excess water was dried with the aid of an absorbent paper. Next, a new measurement of the specimen’s length was performed (Lfinal).
The dimensional change of the specimens was calculated based on the following formula:

Lfinal is equivalent to the length of the specimen after 24 hours and 30 days, and Linitial corresponds to the initial length.
Solubility
For each experimental group, 6 specimens were fabricated using Teflon rings (7.75 inner diameter × 1.5 mm in thickness), according to the specimens’ dimensions proposed by Carvalho-Junior et al. [22]. In each Teflon ring, a perforation was created using a No.2 spherical carbide bur to pass a nylon thread through, which was used for the specimen suspension during the solubility test. The rings were filled with the experimental cements and the sets were kept in an oven at 37°C and 95% relative humidity for a time equivalent to 3 times the setting time of each cement. Next, cement residues were removed using an abrasive sandpaper #600 (3M) under abundant distilled water irrigation, and the specimens were weighed (WH0) on a precision analytical balance (Mettler-Toledo Ind. E Com. Ltda., Model PE 160). After 24 hours in a desiccator containing silica, each specimen was submitted to a new weighing (WD0). Then, the specimens were placed into a plastic container containing 7.50 mL of distilled water, and kept in an oven at 37°C, according to the Carvalho-Junior et al. [22] methodology. After 30 days, the specimens were removed from their plastic containers, and, after removing the excess water, they were weighed, considering the hydrated weight (WH30d) of the specimen. Then, the specimens were returned to the desiccator for 24 hours and, at the end of this period, they were weighed again (WD30d) (dehydrated weight of the specimen). The solubility of the cements was considered according to the weight loss of each specimen, expressed as a percentage of the weight lost, in comparison with the original weight.
Compressive strength
Teflon molds (3 mm inner diameter × 3.58 mm in thickness) were used to fabricate the specimens, according to the Bernardi et al. [23] methodology. The specimens were kept in an oven at 37°C and 95% relative humidity, for 3 times the setting time of the cements. For each group, 6 specimens were fabricated for 24 hours and 6 for the 30 days of testing (n = 6).
After removing the molds, the specimens were measured with a digital caliper. Abrasive sandpaper #600 (3M) was used to smooth the surfaces of each specimen. Then, the specimens were returned to the oven at 37°C and 95% relative humidity, where they remained for the experimental periods of 24 hours and 30 days.
The compressive strength of the cements was measured in a Universal Testing Machine (Instron 4444; Instron Corp., Canton, MA, USA) (1 mm/min-1). The maximum force applied until the fracture of each specimen was expressed in MPa (N/mm2). The compressive strength was calculated according to the following equation: σ = F / A, where σ is the force in MPa, F is the maximum load during fracture in N and A is the area of the specimen in mm2.
pH analysis
The water in which the specimens were immersed during the dimensional change test was used to measure the pH using a pH meter (PH-1700; Micronal, São Paulo, SP, Brazil) previously calibrated according to buffer solutions (pH 7.0 and 9.0), at a temperature of 25°C ± 2°C. The measurements were performed after 24 hours (L24h) and 30 days (L30d). The pH of the distilled water was measured before the storage of the specimens.
Radiopacity
Twenty-four specimens (n = 6) were fabricated using stainless-steel rings (10 mm inner diameter × 1 mm in thickness), following the ISO 6876 standard [15]. After the time interval referring to 3 times the setting time of the cements, the specimens were removed from the rings and stored in a humid environment at 37ºC for 48 hours. The geometrical factors that might interfere in the formation of the radiographic image were standardized by placing the specimens and the aluminum scale (10-mm step wedge thickness) equidistantly on an occlusal radiographic film (Insight-Kodak Comp., Rochester, NY, USA) sensitivity group ‘‘E.’. The occlusal radiographic film was placed on a device that allowed it to maintain the focus/film distance at 30 cm. The locator cylinder was positioned perpendicular to the standardizing device. Then, the specimens and the aluminum scale were submitted to radiographic images acquisition in an X-ray appliance (Dabi Atlante, Ribeirão Preto, SP, Brazil) with a central opening of 11 mm in diameter, set at 60 kV, 7 mA, 15 pulses/second, exposure time = 0.5 seconds and focal length of 30 cm. Pilot tests were performed to determine the exposure time = 0.5 seconds.
The radiographic films were processed in a radiographic film processor (NDT; Kodak), according to the manufacturer’s guidelines regarding the time and temperature used for the processing method. Next, the radiographic films were scanned (Scanner Hp Scanjet G4050, Hp, Palo Alto, CA, USA) and the images obtained were exported to software (ImageJ, https://imagej.nih.gov/ij/) for analysis of the optical and radiographic density of the images, allowing a comparison between the radiopacity of the cements and the different thicknesses of the aluminum scale. Therefore, a predetermined area (400 pixels) was drawn in 3 different areas on the radiographic image of each specimen. The mean value obtained from the pixel grey scale was obtained using the histogram tool of the ImageJ software. The recorded values were calculated to obtain a single value in mm on the aluminum scale (mmAl), according to the ISO 6876 standard [15].
Statistical analysis
The statistical analysis was performed with the SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). The results of the different tests were statistically compared after validating the normality (the Shapiro-Wilk test, p > 0.05) and homogeneity of variance (the Levene test, p > 0.05) assumptions of the data sets. For the radiopacity values, subsequent statistical analysis was performed with a 1-factor analysis of variance (ANOVA) and complemented by Bonferroni’s post-hoc test. For the other physicochemical properties, the data were analyzed using the ANOVA test, followed by the Tukey and Fisher tests (F test). The significance level was pre-set at 5% (α < 0.05).
RESULTS
SEM, TEM, and EDS analysis
The SEM analysis revealed that the nano-ZnO powder had a cauliflower-like granules aspect (Figure 1A). Under TEM, rounded particles with a nanometric size (average between 30–90 nm), irregular shape, and agglomerated areas were observed (Figures 1B and 1C). The EDS analysis (Figure 1D-F) revealed the main components of the nano-ZnO, such as oxygen (82.3% in weight) and zinc (15% in weight). However, the presence of a few impurities was also noted, such as carbon, beryllium, and silicon. The SEM analysis of the CAC containing conventional-ZnO revealed the presence of a powder with a dense and granular aspect (Figure 2A). Under TEM, particles with micrometric size (between 0.15–0.6 µm) and rectangular shape were noted (Figure 2B and 2C). The EDS analysis (Figure 2D-2F) confirmed the presence of aluminum, calcium, and chlorine (main components of calcium aluminate); oxygen and zinc (components of ZnO used as a radiopacifier); and the presence of carbon and zirconia (impurities).
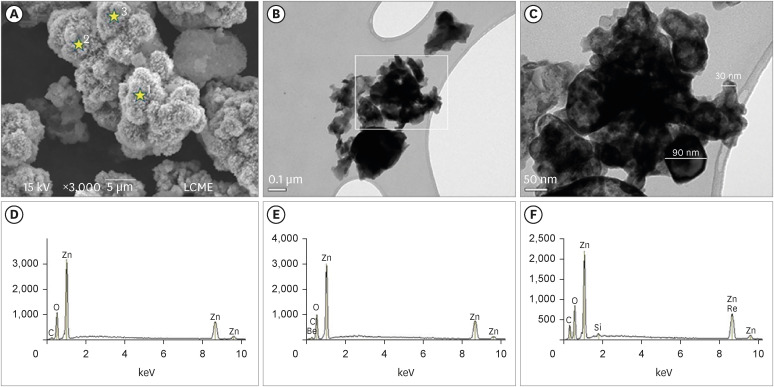
Micrographic images obtained under SEM at ×3,000) (A); and TEM at ×100,000 (B) and at ×250,000 (C) of nano-ZnO powder. It was possible to observe under SEM the cauliflower-like granules aspect of the nano-ZnO (A). Under TEM, spherical particles with irregular shape and agglomerated areas were observed (B). Image (C) with a higher magnification of the highlighted area in (B). Note the presence of particles with a nanometric size (diameter average between 30–90 nm) (C). EDS spectrum (D-F) resulting from the nano-ZnO powder analysis at the points marked with stars in (A): point 1 (D), point 2 (E), point 3 (F). The EDS analysis found the presence of main components, oxygen (82.3% in weight) and zinc (15% in weight), but also the presence of some impurities, such as carbon, beryllium, and silicon.
SEM, scanning electron microscopy; TEM, transmission electron microscopy; nano-ZnO, nanoparticulated zinc oxide; EDS, energy-dispersive X-ray spectroscopy.
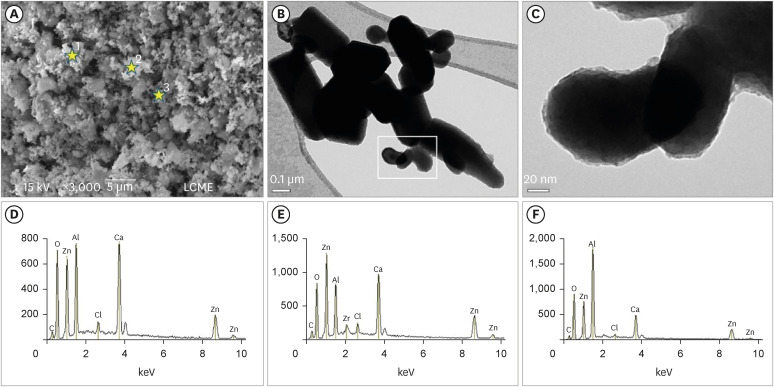
Micrographic images obtained under SEM at ×3,000 (A) and TEM at ×100,000 (B) and ×600,000 (C) of calcium aluminate cement powder containing conventional-ZnO. Note (SEM image) the dense and granular aspect of the cement powder (A). Under TEM, particles with micrometric size and rectangular shape were also noted (B). Greater magnification (C) of the highlighted area in (B). Observe particles with an average size between 0.15–0.6 µm (C). EDS spectrum (D-F) resulting from the material analysis at the points marked with stars in (A): point 1 (D), point 2 (E), point 3 (F). The EDS analysis confirmed the presence of aluminum, calcium, and chlorine (main components of calcium aluminate); oxygen and zinc (components of ZnO used as a radiopacifier); and the presence of carbon and zirconia (impurities).
SEM, scanning electron microscopy; TEM, transmission electron microscopy; nano-ZnO, nanoparticulated zinc oxide; EDS, energy-dispersive X-ray spectroscopy.
Physicochemical properties
The mean values obtained in the different tests for each experimental cement may be seen in Figure 3 (radiopacity), Table 2 (setting time, compressive strength, dimensional change, and pH), and Table 3 (solubility).
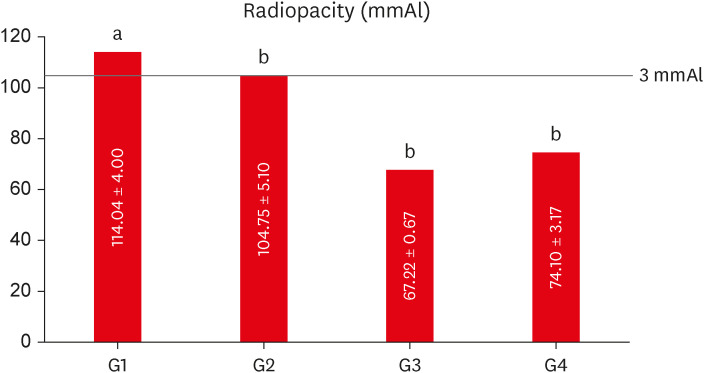
Mean values of radiopacity expressed in aluminum thickness (mmAl) for each experimental group. Different lowercase letters over bars represent statistically significant differences among groups. (One-way analysis of variance, Bonferroni’s test, α = 0.05).
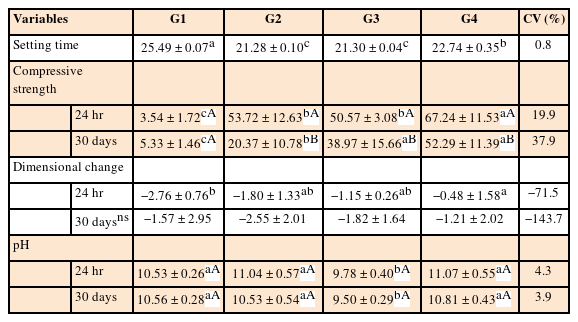
Mean values and standard deviations for the setting time (minutes), compressive strength (MPa), dimensional change (%) and pH for each experimental group
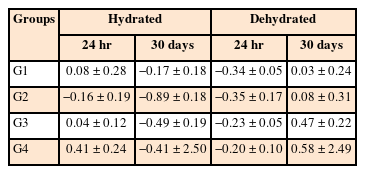
Mean values and standard deviations for the solubility (%) in the periods of 24 hours and 30 days for each experimental group
The addition of nano-ZnO to CAC significantly reduced the setting time of the experimental cements (G2 = 21.28, G3 = 21.30, and G4 = 22.74) (p < 0.05) in comparison with the control group (G1 = 25.49). G2 and G3 had the shorter setting time and did not differ from each other (p > 0.05). The setting time of G2 and G3 were significantly shorter than G4 (p < 0.05).
In the dimensional change test, all experimental groups had a statistically significant difference in relation to the 24-hour period (p < 0.05), where G4 (10% conventional-ZnO + 5% nano-ZnO) presented significantly less dimensional change than the control group (G1) (p < 0.05). However, during the 30 day-period of analysis, the difference among groups was not significant. Except for G4 at the 24-hour period, the dimensional change was not in accordance with specification No. 57 of the ANSI/ADA [5], as the specimens in the other groups and periods had contractions greater than 1%.
The solubility test showed no significant difference among the groups after 24 hours and 30 days, regardless of the hydrated or dehydrated condition of the specimens. In addition, the values were within the limit established by specification No. 57 of the ANSI/ADA [5], which should not exceed a value of 3% mass loss.
As for the compressive strength, after 24 hours and 30 days, all groups containing nano-ZnO had mean values statistically greater than G1 (p < 0.05). When comparing the groups containing the nanoparticles, G4 had higher compressive strength than G2 and G3, in both periods of analysis (p > 0.05). In general, the compressive strength after 30 days was significantly lower when compared to the 24-hour period for the groups containing nano-ZnO (p > 0.05). However, for G1, there was statistically no significant difference between either of the periods of analysis (p < 0.05).
In the pH analysis, all experimental cements promoted an alkaline medium, which was maintained until the final period of 30 days. G3 had the lowest pH values, with a significant difference in comparison with the other groups (p > 0.05), for both periods (24 hours and 30 days).
The group containing 20% of conventional-ZnO (G1 - control) had the highest radiopacity mean value, with a statistically significant difference in comparison with the other groups (p < 0.05) (Figure 3). When compared to the aluminum scale, only G1 (20% conventional-ZnO) and G2 (15% conventional-ZnO + 5% nano-ZnO) had values equivalent to the 3.0 mm of the Al scale (p > 0.05), as recommended by the ISO 6876 standard [15].
DISCUSSION
This study evaluated the effect of adding different proportions of nano-ZnO on the physicochemical properties of CAC in comparison with CAC containing 20% conventional-ZnO. Based on the results obtained, it could be stated that the null hypothesis tested was rejected since the addition of nano-ZnO affected the physicochemical properties of CAC.
CAC is a hydraulic binder with high-mechanical resistance, proper rheological characteristics, and a fast hydration rate in comparison with other mineral aggregate-based cements [7]. It is highly consolidated in the scientific literature that the physicochemical properties of mineral aggregate-based cements, such as CAC, are improved by the addition of nanomaterials to their composition [2324]. For this reason, different proportions of nanoparticles of ZnO were incorporated into CAC in the present study [1718].
Nanomaterials usually have ultra-small dimensions (1 to 100 nm in size) [25]. SEM and TEM were used to characterize the shape and size of the materials tested in the present study. Micrographic images obtained under SEM of nano-ZnO powder showed the aggregation and cauliflower-like granules aspect of the nano-ZnO [2627]. The particle size of the zinc oxide power was verified by TEM under magnification of ×100,000 to ×600,00 and revealed the nanometric size of the nano-ZnO used, with particles ranging from 30 to 90 nm in diameter. TEM was used because it presents high-resolution images, which provide informative details regarding the atomic scale-like morphology, aggregation state, and particle distribution [28]. Other studies have also verified the particle size and shape of nanomaterials using TEM analysis, which is considered one of the most appropriate microscopic techniques for this type of evaluation [2326272829]. Miri et al. [26] verified synthesized ZnO nanoparticles by TEM analysis, which indicated particles with hexagonal shapes and average size (measured diameter) between 50–80 nm. Also, Singh et al. [27] demonstrated the formation of stable ZnO nanoparticles, mostly spherical particles with a particle size ranging from 35 to 80 nm, very similar to what was verified in the present study. The characterization of this material was accomplished by EDS analysis, which identified the element composition and linkage of metabolites and facilitated the interpretation of nanoparticle distribution [28]. Our results demonstrated the main presence of oxygen (82.3% in weight) and zinc (15% in weight) in the nano-ZnO power. These results corroborate with other studies that used the same analysis [2627].
The micrographic images obtained under SEM and TEM of CAC powder containing conventional ZnO demonstrated the dense and granular aspect of the cement powder [30]. Under TEM, particles with micrometric size and rectangular shape were also noted. The EDS analysis confirmed the presence of aluminum, calcium, and chlorine (main components of calcium aluminate); oxygen and zinc (components of ZnO used as a radiopacifier); and the presence of carbon and zirconia (impurities). These findings also were shown in another study corroborating the present results [30].
According to Cao et al. [25], particles of nanometric size affect the physical characteristics of cementitious materials, especially the dilution and nucleation process during setting. Because of their large surface area to volume ratio, even when small quantities of nanoparticles are added to mineral aggregate-based cements, a significant effect on the hydration process may occur [1931]. Therefore, the proportion of radiopacifier in the composition of a mineral aggregate-based cement plays a fundamental role in its microstructure [10]. For this reason, in the present study, in addition to the groups containing 20% of the volume by weight, experimental groups with lower concentrations (15% by weight) of radiopacifiers were evaluated. However, the radiopacity values for these groups were lower than recommended by the ISO 6876 standard [15]. Adequate radiopacity values were only achieved by the groups containing 20% conventional-ZnO (control) and 15% conventional-ZnO + 5% nano-ZnO. Therefore, G3 (12% conventional-ZnO + 3% nano-ZnO) and G4 (10% conventional-ZnO + 5% nano-ZnO) did not fulfill the ISO 6876 standard requirement.
Radiopacity is one of the most important physical properties of materials used for endodontic purposes [14]. According to the ISO 6876 standard, their radiopacity must be greater than 3.0 mm in relation to the aluminum scale to be properly differentiated from bone and dental tissues [15]. Our results demonstrated that the lower radiopacity values obtained from specimens of the G3 and G4 do not allow them to be radiographically visualized and differentiated from adjacent anatomical structures [1415].
Camilleri and Gandolfi [13] have reported that MTA only reached adequate radiopacity when 30% conventional-ZnO was added to their composition, demonstrating that chemical elements with a high atomic number are necessary to obtain adequate radiopacity. Conversely, large additions of radiopacifiers should be avoided, as they may compromise the physicomechanical properties of the cements, and consequently, their clinical performance [10].
Aguilar et al. [14] demonstrated that the addition of 20% conventional-ZnO to CAC was sufficient to reach an adequate radiopacity, according to the ISO 6876 standard [15]. The same might be observed in the present study, in which the highest mean radiopacity value was obtained by the control group (20% conventional-ZnO), in comparison with the other experimental groups. The conflicting results between these studies may be explained by the different natures of calcium silicate-based cements and CAC [1314]. Although both cements are mineral aggregates of hydraulic setting, important differences in the composition may influence their radiopacity, despite the use of the same radiopacifying agent [10]. Despite having a relatively low atomic number (Z = 30) in comparison with other chemical elements, zinc was able to promote greater radiopacity to CAC when compared to the calcium silicate cement, even in a lower concentration [10].
Several studies have already reported that the addition of nanoparticulate compounds to different materials may improve some of their physicochemical properties [1821]. Versiani et al. [18] demonstrated that the addition of 25% of nano-ZnO to a zinc oxide eugenol-based sealer improved its dimensional stability, flowability, radiopacity, and solubility. In the present study, only the group containing 15% conventional-ZnO + 5% nano-ZnO (G2), of those who used nanoparticles, reached the minimum radiopacity values suggested by the ISO 6876 standard [15]. Despite the satisfactory result presented by G2, its radiopacity was statistically lower than the control group (G1).
The setting time of endodontic cements is not standardized and it may significantly range according to their manufacturer and chemical nature [18]. In this study, the addition of nano-ZnO to CAC promoted a significant decrease in setting time compared to the control group (G1). The reduced setting time is a desirable characteristic for repairing cements, as it facilitates its clinical applicability and decreases its toxicity when in contact with the periodontal and periapical tissues [23]. This finding corroborated the studies by Camiletti et al. [24], in which the incorporation of calcium carbonate nanoparticles (nano-CaCO3) into Portland cement also reduced the setting time. Similar results were also observed in the study by Bernardi et al. [23], with lower setting time values after the addition of nano-CaCO3. According to this study, the addition of nanoparticles to the cement increases the contact points within the material particles, which, consequently, accelerates the cement setting time.
The dimensional stability of cement is a very important property, since the lack of adaptation of the cement to the dentin walls may lead to gap formation and bacterial microleakage, compromising the endodontic treatment [24]. Our results showed that the groups containing nano-ZnO had lower values of dimensional change at the 24 hour-period. As reported by Camiletti et al. [24], the addition of nanoparticles to cement promotes acceleration in the hydration process. The greater amount of hydrated cement may reduce water absorption and, thus, maintain a stable volume [24]. After 30 days, the difference among the experimental groups was not significant. It is valid to emphasize that, except for G4 at the 24-hour period, the mean values of dimensional change observed in each group, regardless of the period of analysis, were not in accordance with the specification No. 57 of the ANSI/ADA, as they had a contraction greater than 1% [5].
The solubility must be as low as possible (lower than 3% of its mass), since greater solubilization may lead to the formation of voids and empty spaces, compromising the microstructure of a cement, and consequently, its sealing ability and mechanical resistance [5]. As it relates to the solubility test, the authors opted for the methodology described by Carvalho-Junior et al. [22], which used smaller specimens than suggested by specification No. 57 of the ANSI/ADA [5]. In the present study, the solubility test did not show statistically significant differences among groups, regardless of the period evaluated. In addition, the results were compatible with those proposed by specification No. 57 of the ANSI/ADA [5].
As for the compressive strength, greater resistance was observed after 24 hours in the groups containing the nanoparticles, in comparison with the control group. After 30 days, despite being lower than the other experimental groups, the control group maintained its mechanical resistance, while the other groups presented a significant decrease in their compressive strength values. These results are similar to those found by Camiletti et al. [24], in which the authors concluded that the addition of nano-CaCO3 to Portland cement caused an increase in the initial compressive strength. Furthermore, such increase in the mechanical resistance was directly proportional to the amount of additives incorporated into the cements. In this same study, after 28 days of analysis, all groups had a reduction in compressive strength when compared to the control group, similar to the results obtained in the present study. In the study by Guerreiro-Tanomaru et al. [17], the addition of ZrO or ZnO nanoparticles to Portland cement, drastically reduced the compressive strength of this material. The difference in the results obtained may be related to the different methodologies used, such as the use of two different radiopacifiers and their interaction. In the study by Garcia et al. [8], the compressive strength of CAC containing bismuth oxide (Bi2O3) (20% by weight) as a radiopacifier was higher than the values obtained by the control group in the present study. However, in addition to the difference in the radiopacifiers used (Bi2O3 versus ZnO), there was a crucial difference in the tests performed in both studies. In the present study, the specimens were immersed in distilled water for 24 hours and 30 days before the compressive strength test, while in the study by Garcia et al. [8], the cement was submitted to the test shortly after setting.
The alkaline pH promoted by mineral aggregate-based cements and its maintenance at a higher level is fundamental to allow the deposition of mineralized tissue, as well as the assurance of an effective antimicrobial action of this type of material [17]. According to Estrela et al. [32], pH values close to 12 have the ability in inhibiting resistant microorganisms. In the present study, the experimental cements promoted an alkaline pH at the 24 hour-period, which remained at the same levels after 30 days. G3 (12% conventional-ZnO + 3% nano-ZnO), despite presenting an alkaline pH, had lower values than the other groups. The pH observed in the control group was higher than described by Pires-de-Souza et al. [16], which used the same proportion of ZnO (20%) as a radiopacifier for CAC. This fact may be explained by the different methodologies used in both studies. In the present study, the pH reading was performed in the water in which the specimens were stored during the dimensional change test, while in the study by Pires-de-Souza et al. [16], the water was replaced at each pH check.
Nanotechnology has enormous potential in the dentistry field. The addition of nanoparticles to repairing cements, such as CAC, affects their performance and technical functionality, and it is highly recommended when compared to their conventional counterparts. The promising results found in this study should inspire further tests, especially regarding the biological properties of CAC containing different nano-ZnO ratios.
CONCLUSIONS
Within the limitations of a laboratory study, it was possible to conclude that the nanoparticles of zinc oxide positively affected some physicochemical properties of the CAC, especially the dimensional change, setting time, and compressive strength. The addition of nanoparticles to CAC formulation is a feasible option, as it may improve its clinical performance.
ACKNOWLEDGEMENTS
The authors would like to thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) and the Federal University of Santa Catarina (UFSC) for the financial assistance to carry out this research. The authors would like to also thank the Central Laboratory of Electronic Microscopy (LCME) of the Federal University of Santa Catarina for the SEM, TEM, and EDS equipment support.
Notes
Funding: The authors would like to thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) and the Federal University of Santa Catarina (UFSC) for the financial assistance to carry out this research.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Garcia LFR, da Rosa AF, Amaral TS.
Data curation: Paz Dotto, Goulart TS, Teixeira CS, Garcia LFR.
Formal analysis: Bortoluzzi EA, Teixeira CS, Garcia LFR.
Funding acquisition: Teixeira CS, Garcia LFR.
Investigation: da Rosa AF, Amaral TS, Goulart TS, Paz Dotto, Rossetto HL.
Methodology: da Rosa AF, Amaral TS, Goulart TS, Paz Dotto, Rossetto HL.
Project administration: Teixeira CS, Garcia LFR.
Resources: Bortoluzzi EA, Teixeira CS, Garcia LFR.
Software: Teixeira CS, Garcia LFR.
Supervision: Bortoluzzi EA, Teixeira CS, Garcia LFR.
Validation: Goulart TS, Paz Dotto, Rossetto HL.
Visualization: Goulart TS, Paz Dotto, Rossetto HL.
Writing - original draft: da Rosa AF, Amaral TS.
Writing - review & editing: Bortoluzzi EA, Teixeira CS, Garcia LFR.




