Stem cell-derived exosomes for dentin-pulp complex regeneration: a mini-review
Article information
Abstract
This mini-review was conducted to present an overview of the use of exosomes in regenerating the dentin-pulp complex (DPC). The PubMed and Scopus databases were searched for relevant articles published between January 1, 2013 and January 1, 2023. The findings of basic in vitro studies indicated that exosomes enhance the proliferation and migration of mesenchymal cells, as human dental pulp stem cells, via mitogen-activated protein kinases and Wingless-Int signaling pathways. In addition, they possess proangiogenic potential and contribute to neovascularization and capillary tube formation by promoting endothelial cell proliferation and migration of human umbilical vein endothelial cells. Likewise, they regulate the migration and differentiation of Schwann cells, facilitate the conversion of M1 pro-inflammatory macrophages to M2 anti-inflammatory phenotypes, and mediate immune suppression as they promote regulatory T cell conversion. Basic in vivo studies have indicated that exosomes triggered the regeneration of dentin-pulp–like tissue, and exosomes isolated under odontogenic circumstances are particularly strong inducers of tissue regeneration and stem cell differentiation. Exosomes are a promising regenerative tool for DPC in cases of small pulp exposure or for whole-pulp tissue regeneration.
INTRODUCTION
The dental pulp is the only soft tissue in the tooth and is surrounded by mineralized tissues (enamel, dentin, and cementum). It is composed of cells including fibroblasts, odontoblasts, immune cells, extracellular matrix components, blood vessels, and nerves. It provides nutrition for teeth; forms primary, secondary, and tertiary dentin; transmits sensory information; and provides immunoprotection [1]. Dentin is a specialized calcified tissue covered by the enamel in the crown and the cementum in the root that surrounds the dental pulp as it forms the pulp horns, chamber, and root canals [2]. As dentin and pulp tissues originate from the dental papilla and have interrelated functions, they form a structure called the dentin-pulp complex (DPC), which maintains the normal function and structural integrity of the entire tooth [34]. If the outer shell of the mineralized tissues is irreversibly damaged by dental caries or traumatic injury, the pulp becomes exposed and pulp vitality is compromised [45]. Pulp exposure facilitates bacterial infection that leads to inflammation and necrosis of the pulpal tissue; this ultimately affects the lifespan of the tooth, unfavorably impacting the patient’s oral health [6].
In clinical situations, 2 pathological conditions involving the dental pulp are most common. Initially, when the dental pulp is potentially inflamed and still vital, the goal is to maintain its vitality and regenerate the DPC to preserve the pulp and reestablish a protective mineralized barrier [7]. The dental biomaterials used in this strategy should be biocompatible and have adequate sealing capacity to allow pulp healing/regeneration [8]. Pulp capping is the main therapeutic method aimed to maintain the pulp vitality and stimulate pulp tissue repair and dentin bridge formation; in this therapy, a biocompatible material is placed directly over the exposed pulp (direct pulp capping) or the unexposed pulp (indirect pulp capping) [910]. Currently, several materials are used for pulp capping, including calcium hydroxide paste, mineral trioxide aggregate, and glass ionomer resin [510]. However, the failure rate after pulp capping is high due to the presence of tunnel defects in the newly formed dentin bridge [11].
The other commonly encountered condition is the progression of inflammation within the pulp tissue to acute, chronic, or chronic hyperplastic irreversible pulpitis. The anatomical constraints of the pulp tissue, such as a low-compliance environment and lack of collateral circulation, may worsen the situation and can quicken the complete loss of the pulp vitality, which is followed by necrosis, root canal infection, and periapical disease [12]. In these circumstances, the pulp is not amenable to treatment and should be removed by pulpectomy or extraction [8]. Root canal treatment depends on the complete removal of the pulp tissue, mechanical debridement, disinfection, and filling of the root canal with an inert synthetic material. While root canal treatment can efficiently preserve dentin, at least to some extent, and control infection, it has several disadvantages, such as postoperative tooth fracture and prevention of apical closure in immature teeth [7131415]. Therefore, more attention has been directed toward strategies to regenerate a new vital tissue biomimetically resembling the pulp tissue [8].
Regenerative endodontic procedures are biological approaches involving tissue engineering that are considered effective treatments for immature necrotic permanent teeth. These include revascularization, partial pulpotomy, and apexogenesis [16]. In a previous study, when regenerative approaches were used, immature dog teeth with apical periodontitis survived, and the differentiation potential of the apical papilla stem cells (SCs) was retained. In addition, sodium hyaluronate: chitosan or pectin: chitosan scaffolds that were added to the blood clot did not improve the histologic evidence of the regenerated DPC or the formation of new mineralized tissues along the root canal walls [17]. Clinically, regenerative endodontic procedures have been found to support survival and the continued potential differentiation of the apical papilla stem cells after endodontic infection [1819].
Pulp healing is a complex process that is dependent on many factors, including the extent of inflammation, type and location of the injury, and age of the tooth. As in development, many cellular and molecular cascades occur in this process. As mentioned earlier, the main purpose of the DPC is to maintain the protective covering of dentin through self-protective and homeostatic mechanisms, as the pulp tissue can ameliorate secondary or tertiary dentin formation to shield the pulp from external irritants or stimuli. In the case of minor injury, healing consists of reactionary dentin formation with the simple induction of dentinogenesis by existing odontoblasts. However, with excessive tissue injury, more complex defenses occur involving dental pulp stem cell (DPSC) migration and their differentiation to odontoblast-like cells; this forms another type of tertiary dentin termed reparative dentin [820]. The regulation of odontoblast activity is fundamental for DPC healing and regeneration, and the mechanisms involved in this process must be understood [8]. The mitogen-activated protein kinase (MAPK) pathway appears to be vital for odontoblast stimulation and may offer a key objective for therapeutic intervention [21]. Platelet-derived growth factor and transforming growth factor beta 1 (TGF-β1) also have well-established roles in promoting odontoblast differentiation during primary dentin formation and in secondary or tertiary dentinogenesis, which may be attributed to their regulatory role in MAPK pathway activity [82223]. Figure 1 is a diagrammatic representation of the hemostatic, early/late inflammatory, and regenerative phases of pulp self-healing mechanisms.
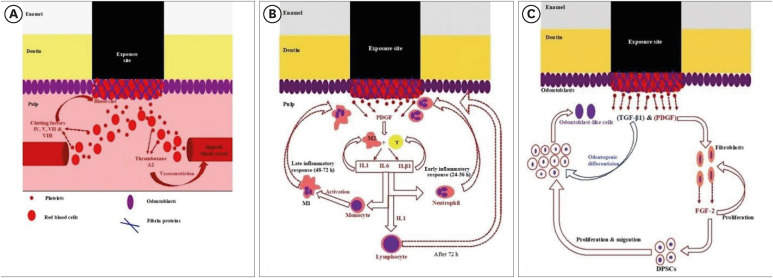
Diagrammatic representation of (A) hemostasis, (B) early/late inflammatory, and (C) regenerative phases of pulp self-healing mechanisms.
IL, interleukin; TGF-β1, transforming growth factor beta 1; PDGF, platelet-derived growth factor; DPSC, dental pulp stem cell.
SC-based tissue engineering has been broadly studied for DPC regeneration, including in clinical trials [2425]. While SC-based therapies are effective, they are expensive, commercially challenging, and carry immunological concerns; in addition, the cell behavior inside the body cannot be predicted [26]. Since the regenerative effect of SCs is related to exosome release rather than the SCs themselves, researchers of regenerative medicine are increasingly turning their focus to exosomes secreted by the cells. The use of exosomes may produce considerable advantages over the direct use of SCs due to their easier separation, handling, and preservation; lower immunogenicity; and better safety profile [2728]. Additionally, exosomes could exhibit the same advantages associated with SCs by controlling the apoptosis, migration, differentiation, and proliferation of target cells [29]. Thus, exosomes could be used as a biomimetic regenerative tool to induce SC differentiation and allow cell recruitment [6], whether from within the pulp tissue or from other sites of the body [8]. This mini-review was designed to present an overview of the use of exosomes as a new line of treatment in DPC regeneration.
MATERIALS AND METHODS
A PubMed and Scopus search was performed for relevant articles published between January 1, 2013 and January 1, 2023. The reference lists of all retrieved articles were assessed to identify additional relevant studies. Each included study was independently assessed by at least 2 authors (Zaher A and Mansour A), and rating decisions were based on the consensus of the principal author (Grawish M). The search strategy was (Exosomes) AND (Dentin Pulp Complex), (Exosomes) AND (Dentin), and (Exosomes) AND (Pulp). The inclusion criteria for the study selection were 1) all types of in vivo and in vitro experimental studies, 2) studies performed on humans and other species, and 3) language restricted to English. The exclusion criteria were 1) studies performed on DPC using conditioned media, 2) qualitative and/or quantitative reviews, 3) commentaries, 4) letters to the editor, and 5) books and book chapters.
RESULTS
The initial search of the PubMed and Scopus databases yielded 235 articles. The search of the references of related articles resulted in 9 articles. In total, 244 articles were identified. After filtering, 119 articles were excluded, and after the eligibility criteria were applied to the remaining 30 articles, 14 studies were included in this review. Details of included studies are shown in Tables 1 and 2. A flow chart for the selection process is presented in Figure 2.
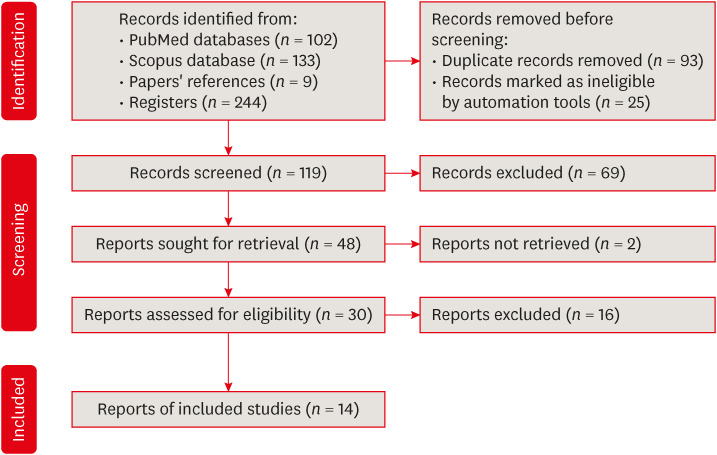
Preferred reporting items for systematic reviews and meta-analyses flow diagram for article selection.
In vitro and in vivo studies
Several basic in vitro and in vivo studies were performed to evaluate the role of exosomes in different aspects of DPC regeneration (Tables 1 and 2). Unfortunately, no evidence yet exists from any human clinical trials.
Source of exosomes
Exosomes were derived from human (h) primary SCs in the form of hDPSCs, odontogenic-differentiated hDPSCs, lipopolysaccharide (LPS)-stimulated hDPSCs, human stem cells from the apical papilla (hSCAPs), stem cells from human exfoliated deciduous tooth (hSHED) aggregates, and human umbilical cord mesenchymal stem cells (hUCMSCs). Cell lines from animal sources were also used for exosome isolation as immortalized murine odontoblast cell lines (mDPCs) and rat epithelial root sheath of Hertwig (HERS) cells [56283031323334353637383940].
In vitro studies
After the exosomes were isolated and characterized, their potential effects were studied using primary hDPSCs, LPS-stimulated hDPSCs, human bone marrow mesenchymal stem cells (hBMSCs), human umbilical vein endothelial cells (hUVECs), and a cell line of human Schwann cells [562830313235373839]. Primary cells from animal sources were also used for the evaluation of exosomes, including rat BMSCs, rat DPSCs, and mouth regulatory T (Treg) cells [33343640].
Regarding human-derived exosomes, in vitro, exosomes derived from hDPSCs cultured under growth conditions triggered the P38 MAPK pathway and increased the expression of genes for odontogenic differentiation of hDPSCs and hBMSCs [6]. They upregulated odontogenic gene expression involving bone sialoprotein, dentin sialophosphoprotein, and vascular endothelial growth factor (VEGF) and consequently improved the mineralization activity of hDPSCs [5]. In addition, these exosomes attracted and improved the proliferation of hBMSCs, especially when fibrin gel was used as a delivery system, as it enhanced the ameliorative effects of the exosomes [28]. Likewise, exosomes positively impacted the growth of hUVECs in monolayer culture and 3-dimensional co-culture of hDPSCs and hUVECs seeded on fibrin gels. In less than 7 days, the fibrin gels facilitated the formation of a capillary-like structure by increasing the expression of VEGF and promoting the deposition of collagen types I, III, and IV [32]. The exosomes promoted the proliferation of hUVECs, enhanced the expression of proangiogenic factors, and induced capillary tube formation through the p38 MAPK signaling pathway; therefore, hDPSC-exosomes are considered a promising regenerative tool in pulp regeneration [30]. At the same time, these exosomes were found to alleviate the inflammatory response of LPS-induced hDPSCs by decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines [39].
Comparatively, exosomes isolated from hDPSCs cultured under odontogenic/osteogenic conditions were better enhancers of hDPSC differentiation than those cultured under growth conditions, as the exosomal microRNA of the former enhanced odontogenic cell differentiation via the TGF-β1/Smad signaling pathway and downregulation of latent TGF-β binding protein 1 [31]. In another study, exosomes derived from LPS-stimulated hDPSCs triggered the angiogenic potential of hUVECs by promoting proliferation, migration, and capillary tube formation with increased expression of VEGF and kinase insert domain-containing receptor [35]. Also, exosomes isolated from the supernatant of hDPSCs or LPS-prestimulated hDPSCs promoted the proliferation, migration, and odontogenic differentiation of Schwann cells [38]. Exosomes derived from hDPSCs cultured with or without LPS were found to modulate rat BMSC differentiation, angiogenesis, migration, and proliferation [36]. In addition, exosomes derived from hSHED aggregates promoted endothelial cell differentiation and enhanced the proangiogenic/angiogenic capability of hUVECs by regulating the TGF-β/Smad2/3 signaling pathway [37]. Likewise, exosomes derived from hUCMSCs were superior to those derived from hDPSCs in ameliorating the LPS-induced inflammation of hDPSCs by decreasing pro-inflammatory cytokines and increasing anti-inflammatory cytokines [39]. Moreover, hSCAP-derived exosomes enhanced the dentinogenesis capacity of rat BMSCs with the increased gene and protein expression of dentin sialoprotein and the formation of mineralized nodules [34]. They also promoted the Treg conversion of C57BL/6 in female mice [40].
Regarding animal-derived exosomes, the mDPCs had the same upregulatory effects as hDPSC-derived exosomes for bone sialoprotein, dentin sialophosphoprotein, and VEGF odontogenic gene expression and also improved the mineralization activity of hDPSCs [5]. In addition, exosomes derived from rat HERS cell lines promoted the migration and proliferation of rat DPSCs and induced their odontogenic differentiation via the activation of the Wingless-Int/β-catenin signaling pathway, capillary tube formation, and neural differentiation [33].
In vivo studies
Tooth root slices were subcutaneously implanted on the backs of nude mice for 2 weeks. Each root slice was filled with primary hDPSCs embedded within either control material or exosomes derived from hDPSCs and incorporated within collagen membranes. The exosomes triggered the regeneration of dentin-pulp–like tissue, and exosomes isolated under odontogenic conditions were better inducers of SC differentiation and tissue regeneration than exosomes isolated from cells cultured in growth medium [6]. Also, subcutaneous implantation of root fragments containing rat BMSCs and hSCAP-exosomes in immunodeficient mice produced dentin-pulp–like tissue with newly formed dentin that was clearly observed in the SCAP-exosome group. Odontoblasts were polarized, columnar, and arranged in an ordered pattern at the junction of the pulp and predentin, and their processes extended into the dentinal tubules [34]. Likewise, subcutaneous transplantation of tooth fragments containing SHED cell aggregates with or without GW4869 (a sphingomyelinase inhibitor used for blocking exosome generation)/SHED aggregate-derived exosomes into the backs of the mice for 12 weeks considerably improved angiogenesis and pulp tissue regeneration, especially when the SHED aggregate-derived exosomes were used [37]. Similarly, subcutaneous implantation of exosomes derived from hDPSCs or immortalized mDPCs that were immobilized onto poly (L-lactic acid) nanofibrous scaffolds and incorporated into 1.0-mg polymer microspheres (poly [lactic-co-glycolic acid] [PLGA]-polyethylene glycol [PEG])-PLGA induces DPSC differentiation with no inflammation and minimal fibrous capsule formation [5]. Also, exosome-like vesicles derived from HERS cells transplanted subcutaneously into nude mice or into rat renal capsules in combination with collagen gel and DPSCs isolated from unerupted first molars of 1- to 3-day-old postnatal Sprague-Dawley rats triggered regeneration of dentin-pulp–like tissue that consisted of blood vessels as well as neuronal and reparative dentin-like tissues [33].
Fortunately, when a rat molar pulp-capping model was used, the amphiphilic synthetic polymeric vehicle synthesized from poly (L-lactic acid) nanofibrous scaffolds incorporating microspheres of PLGA-PEG-PLGA triblock copolymers and encapsulating hDPSCs or mDPC-derived exosomes resulted in the acceleration of tertiary dentin and bridge formation without signs of bacterial infection in comparison with using glass-ionomer cement separately, after a 6-week examination period [5]. In comparison, implantation of exosomes derived from hDPSCs cultured with or without LPS into a rat pulpless root canal containing PuraMarix peptide hydrogel and BMSCs enhanced the formation of regenerated pulp tissue resembling normal dental pulp with higher efficiency associated with exosomes derived from hDPSCs preconditioned with LPS [36].
CONCLUDING REMARKS
Exosomes are lipid-bilayered cell-derived membranous vesicles of nanoparticle scale [41]. They are produced through multivesicular exocytosis [42] and transport various biomolecules including nucleotides, microRNAs (miRNAs), proteins, and lipids [43]. These cargos are transported to recipient cells by binding to surface receptors or through endocytosis (Figure 3). They affect fundamental cellular processes including lineage-specific differentiation, migration, proliferation, and apoptosis [2944]. The isolation of exosomes is challenging, and to date, no optimal protocol exists to isolate them with absolute precision [45].
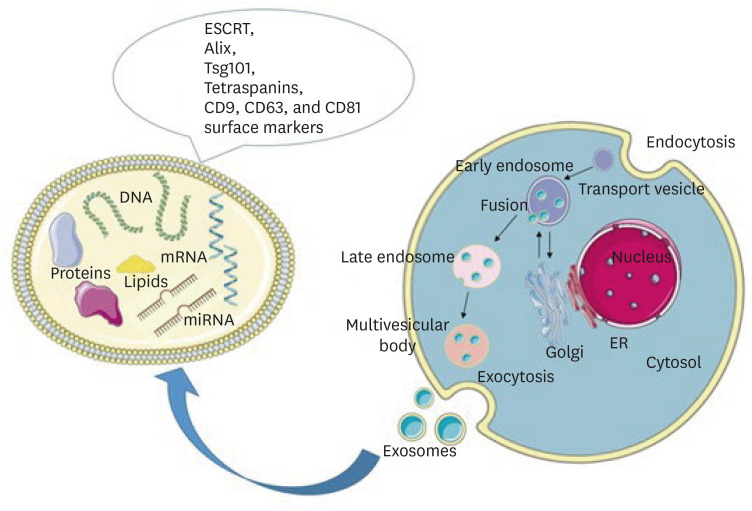
Schematic representation of exosome biogenesis and molecular cargo. Exosomes are formed through the inward budding of the endosomal membrane resulting in the formation of early endosomes, late endosomes, and multivesicular bodies (MVBs). Upon fusion of MVBs with the plasma membrane, exosomes are released into the extracellular space. The molecular cargo of exosomes consists of lipids, DNA, mRNA, microRNA, and proteins. On their surface, they carry the RNA ESCRT, Alix, Tsg101, and tetraspanins CD9, CD63, and CD81 markers. Parts of the figure were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
ESCRT, endosomal sorting complex required for transport.
Despite the difficulty of isolating exosomes, substantial efforts have been made to determine the exosome content that results from exosome biogenesis. The most abundant component of the exosome contents is non-coding RNAs (17–24 nucleotides) termed miRNAs, which regulate gene expression [29]. miRNA cargo may be diagnostic and prognostic of some diseases, as the components of exosomes can depend on disease states [29]. Exosomes also have therapeutic potential in tissue regeneration, wound healing, and oncotherapy. In addition, they can be formulated for use as drug-delivery nanovehicles [46]. The age of the SC donor affects the biological properties of the exosomes; for example, exosomes derived from SHED showed higher levels of miRNA related to osteogenesis and angiogenesis and a higher proliferation rate than those derived from permanent teeth [47].
THERAPEUTIC EFFECTS OF STEM CELL-DERIVED EXOSOMES IN DPC REGENERATION
Exosomes can alter cellular activities related to inflammation, cellular differentiation, apoptosis, matrix remodeling, and epithelial/mesenchymal transition and interaction [46]. Therefore, the biological evaluation of exosomes in DPC regeneration (Figure 4) should include consideration of neovascularization, neoneuralization, anti-inflammatory and immunomodulatory activity, cell proliferation, cell migration, and lineage-specific cell differentiation [29].
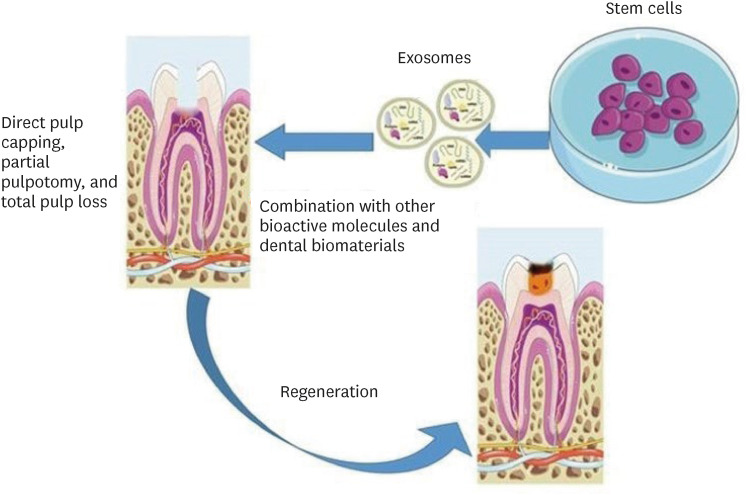
Schematic representation of the suggested methods for the use of exosomes in dentin-pulp complex (DPC) regeneration. Parts of the figure were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
CONCLUDING REMARKS
Exosomes can be considered a promising regenerative tool for DPC or whole pulp regeneration. Exosomes show great potential as a cell-free tissue regeneration strategy, as they can promote migration, proliferation, and odontogenic differentiation of stem cells both in vitro and in vivo. Exosomes can also induce neovascularization and neuralization and modulate immune reactions; in addition, they have anti-inflammatory properties and mediate the interaction between epithelial and mesenchymal cells. They can be incorporated into the polymeric or ceramic bioactive dental materials dedicated to DPC or whole pulp regeneration, as they have powerful and beneficial inductive and stimulatory effects. However, the translational and clinical application of exosomes is still challenging, as the production of exosomal formulations is the major barrier to therapeutic application because of their heterogeneity and low productivity. Exosome formulation requires specific, effective manufacturing practices and quality control protocols to ensure the homogeneity of exosomes and consequent safe use as a therapeutic tool. These protocols include cell cultures, exosome isolation, characterization, modification, and storage. A multidisciplinary team including clinicians, biologists, technologists, and computer professionals working with these small vesicles is necessary to produce significant advances in this promising field.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Grawish ME.
Data curation: Mansour AM, Grawish ME.
Formal analysis: Zaher AR.
Methodology: Saeed MA, Hammouda DA.
Supervision: Zaher AR, Grawish ME, Mansour AM.
Validation: Zaher AR.
Writing - original draft: Grawish ME, Mansour AM, Hammouda DA.
Writing - review & editing: Zaher AR, Grawish ME.




