Effects of CTHRC1 on odontogenic differentiation and angiogenesis in human dental pulp stem cells
Article information
Abstract
Objectives
This study aimed to determine whether collagen triple helix repeat containing-1 (CTHRC1), which is involved in vascular remodeling and bone formation, can stimulate odontogenic differentiation and angiogenesis when administered to human dental pulp stem cells (hDPSCs).
Materials and Methods
The viability of hDPSCs upon exposure to CTHRC1 was assessed with the WST-1 assay. CTHRC1 doses of 5, 10, and 20 µg/mL were administered to hDPSCs. Reverse-transcription polymerase reaction was used to detect dentin sialophosphoprotein, dentin matrix protein 1, vascular endothelial growth factor, and fibroblast growth factor 2. The formation of mineralization nodules was evaluated using Alizarin red. A scratch wound assay was conducted to evaluate the effect of CTHRC1 on cell migration. Data were analyzed using 1-way analysis of variance followed by the Tukey post hoc test. The threshold for statistical significance was set at p < 0.05.
Results
CTHRC1 doses of 5, 10, and 20 µg/mL had no significant effect on the viability of hDPSCs. Mineralized nodules were formed and odontogenic markers were upregulated, indicating that CTHRC1 promoted odontogenic differentiation. Scratch wound assays demonstrated that CTHRC1 significantly enhanced the migration of hDPSCs.
Conclusions
CTHRC1 promoted odontogenic differentiation and mineralization in hDPSCs.
INTRODUCTION
Conventional endodontic procedures include the removal of inflamed pulp and its replacement with synthetic materials. The extrusion of endodontic materials into the periapical tissue can result in a foreign body reaction [1]. Non-vital teeth can no longer sense environmental changes, making it more likely for patients not to notice caries progression. Dental pulp vitality is also advantageous because it preserves the possibility of dentin regeneration to a limited extent. Reparative dentin formation is essential for apical closure and dentinal wall development in immature permanent teeth. If restorative treatment is inadequate, endodontically treated teeth may lose their structural integrity, increasing their vulnerability to masticatory forces [2]. Endodontic therapy often leads to discoloration produced by the filling material, which impairs the esthetics of treated teeth [3]. Maintaining pulp vitality also prevents bacterial infections, thereby reducing the risk of apical periodontitis [45]. Therefore, instead of the currently available methods of endodontic treatment, it would be preferable, if possible, to be able to maintain or restore dental pulp vitality [6]. Vital pulp therapy, which encompasses pulpotomy as well as direct and indirect pulp capping, is a method of treating reversible pulpal injuries. It involves placing a protective dressing on the exposed pulp to prevent further trauma and facilitate repair and healing [7]. Vital pulp therapy has been the focus of substantial attention during the last 40 years, in terms of both its biological and clinical aspects [891011], but the scientific basis of vital pulp therapy remains a topic of debate [1213]. Specific treatment modalities that stimulate biological processes and lead to a reparative dentinogenic response are paramount.
Collagen triple helix repeat containing-1 (CTHRC1) is a 30 kDa secreted protein that promotes migration [14]. It is highly conserved between lower chordates and mammals. CTHRC1 expression was initially observed in rat tissue repair [1516]. CTHRC1 is characteristically expressed by activated fibroblasts associated with wound healing and cancer-activated fibroblasts [1718]. Studies have shown increased expression of CTHRC1 at the protein level in diseased and injured arteries, implying that CTHRC1 may contribute to vascular remodeling as part of tissue repair after injury through its ability to promote cell migration. Angiogenesis is a vitally important and transiently activated response to tissue injury [19]. Studies have recently demonstrated the involvement of CTHRC1 in adipogenesis and bone metabolism [182021].
However, no studies to date have investigated the effect of CTHRC1 in the pulp dentin complex. Dentin is a similar tissue to bone in the body, and pulp tissue is encapsulated with bone. Therefore, angiogenesis is a very important factor for the maintenance of pulp vitality. In this context, the present study investigated the effect of CTHRC1 on odontogenic differentiation and angiogenesis in human dental pulp stem cells (hDPSCs). The hypothesis was that CTHRC1 would induce odontoblastic differentiation, mineralization, and angiogenesis in hDPSCs.
MATERIALS AND METHODS
Cell isolation and culture of hDPSCs
hDPCs were purchased from CEFO Co. Ltd. (Seoul, Korea) and cultured in alpha minimum essential medium (α-MEM 1×; Gibco Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco Invitrogen) and 1% antibiotics (100 mg/mL streptomycin and 100 U/mL penicillin; Gibco Invitrogen) in a humidified atmosphere containing 5% CO2 at 37°C. These incubation conditions were maintained for all cell culture steps. The medium was refreshed at 3-day intervals until the formation of confluent cell monolayers. Subculture was performed after confluence was reached, subculture was performed, and cells from passages 3 to 6 were used for experiments.
CTHRC1 preparation
CTHRC1 human HEK (10 µg) was purchased from Prospec-Tany Technogene Ltd. (Ness Ziona, Israel). The CTHRC1 was diluted with deionized water, creating a working stock solution with an approximate concentration of 0.5 mg/mL, and the lyophilized pellet was allowed to dissolve fully. Aliquots of CTHRC1 were stored at −20°C before use.
Cell viability
CTHRC1’s effect on hDPSC viability was tested using an Ez-Cytox Enhance cell viability assay kit (DoGenBio, Seoul, Korea), following the manufacturer’s manual. hDPSCs were seeded on 96-well plates at a density of 1 × 104 cells per well, followed by incubation for 24 hours. The cells were treated with CTHRC1 at concentrations of 5, 10, or 20 µg/mL for 3 days. Next, 10 µL of Ez-Cytox reagent (DoGenBio) was added to each well, followed by a 2-hour incubation period. In each well, the absorbance at a 450-nm wavelength was measured using a spectrophotometer (Multiskan GO Microplate Spectrophotometer; Thermo Scientific, Waltham, MA, USA).
RNA extraction and polymerase chain reaction (PCR) assay
hDPSCs were seeded on 6-well culture plates at a density of 2 × 105 cells per well, followed by stimulation with CTHRC1 at a concentration of 10 or 20 µg/mL for 5, 7, and 10 days. The TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and the AccessQuick RT-PCR System (Promega, Madison, WI, USA) were used to perform total RNA extraction and complementary DNA synthesis. Quantitative real-time PCR was carried out in triplicate for each sample using the Quanti-Tect SYBR Green PCR Kit (Qiagen, Valencia, CA, USA). Bioneer (Daejeon, Korea) carried out the synthesis of all primers. Relative gene expression was quantified with normalization to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression as a control. The 2−∆∆Ct method was used to analyze gene expression data [22]. The primer sequences are shown in Table 1.
Alizarin red S staining assay
hDPSCs were seeded at 3 × 104 cells per well on 24-well plates and cultured in α -MEM (containing 10% FBS, streptomycin 100 mg/mL, and penicillin 100 U/mL), with a 24-hour incubation. For the experiments involving mineralization, α-MEM supplemented with 10% FBS was added, and the cells were cultured in OM medium (50 µg/mL of ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA) and 10 mmol/L β-glycerophosphate (Santa Cruz, Dallas, TX, USA) before treatment. Cells were divided into a control group (OM was added to the culture medium) and the CTHRC1 group (OM with the addition of CTHRC1 [5, 10, 20 µg/mL respectively] to the culture medium as pretreatment) and cultured for 21 days. The medium (either fresh OM or OM with CTHRC1) was changed every 2 days. After 21 days, the cells were washed with Dulbecco’s phosphate-buffered saline (DPBS). The cells were fixed in 70% ethanol and stained with 300 µL of 2% Alizarin red S staining reagent (LIFELINE Cell Tech, Frederick, MD, USA). A photograph was taken after the staining reagent was removed. A quantitative analysis was performed by measuring absorbance values at a 580-nm wavelength after the addition of 10% cetylpyridinium chloride (pH = 7.0) to the samples.
Scratch wound assay
hDPSCs were seeded on 6-well plates at a density of 7 × 105 cells per well and incubated for 24 hours. The cell monolayer was manually scratched with a 200 µL yellow plastic pipette tip and washed with DPBS. The cells were pre-incubated with CTHRC1 (10 or 20 µg/mL) or without CTHRC1 for a 24-hour period prior to being scratched across the well. The wounded cell monolayer was permitted to heal for 0 or 24 hours, either treated with or without 10 or 20 µg/mL CTHRC1. Images of wound healing were taken using an inverted microscope (Olympus, Tokyo, Japan). The wound closure rate, which was measured and expressed as a percentage of the initial wound length at the time of the scratch using the ImageJ program, was used to quantify cell migration.
Statistical analysis
Each experiment was performed 2 or more times, consisting of independent tests conducted in triplicate. One-way analysis of variance was performed, and the Tukey post hoc test was used to evaluate the statistical significance of differences. GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS version 25.0 (IBM Corp, Armonk, NY, USA) were used for analysis. Differences were considered significant at p < 0.05.
RESULTS
Effects of CTHRC1 on the viability of hDPSCs
The viability of hDPSCs cultured with different concentrations of CTHRC1 (0, 5, 10, and 20 µg/mL) for 48 hours is shown in Figure 1. Cell viability was significantly lower in the group treated with 20 µg/mL CTHRC1 group than in the control group (p < 0.05).
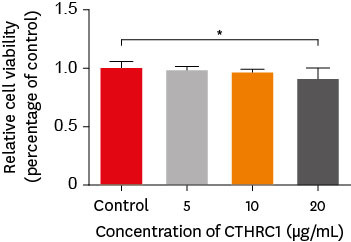
The effect of CTHRC1 on the viability of hDPSCs was measured using the WST-1 assay. Cells were incubated with increasing concentrations of CTHRC1 (0, 5, 10, and 20 µg/mL) for 48 hours. Cell viability was significantly lower in the group that was treated with 20 µg/mL CTHRC1 than in the control group (p < 0.05).
CTHRC1, collagen triple helix repeat containing 1; hDPSCs, human dental pulp stem cells.
Effects of CTHRC1 on mRNA expression of odontogenic and angiogenic markers in hDPSCs
The effects of CTHRC1 on odontogenic differentiation and angiogenesis in hDPSCs were analyzed by quantifying the expression levels of marker genes (dentin sialophosphoprotein [DSPP], dentin matrix protein 1 [DMP-1], fibroblast growth factor 2 [FGF-2], and vascular endothelial growth factor [VEGF]) using real-time PCR. The hDPSCs showed no significant differences in DSPP and DMP-1 mRNA expression levels (Figure 2).
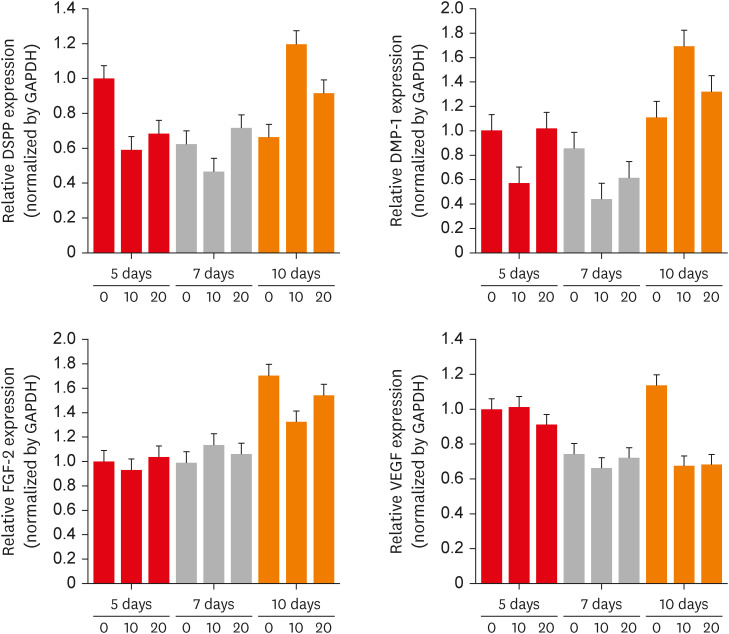
The effects of CTHRC1 on odontogenic differentiation and angiogenesis in hDPSCs. DSPP, DMP-1, FGF-2, and VEGF expression determined using real-time-polymerase chain reaction. Higher mRNA expression of DSPP and DMP-1 was displayed in hDPSCs treated with 10 µg/mL CTHRC1 in a 10-day culture. However, there was no statistically significant difference.
CTHRC1, collagen triple helix repeat containing 1; hDPSCs, human dental pulp stem cells; DSPP, dentin sialophosphoprotein; DMP-1, dentin matrix protein 1; FGF-2, fibroblast growth factor 2; VEGF, vascular endothelial growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
CTHRC1 promoted mineralization in hDPSCs
Alizarin red S staining was carried out on cells stimulated with 0, 5, 10, and 20 µg/mL of CTHRC1 for 21 days to determine the mineralization effect of CTHRC1 in hDPSCs (Figure 3A and 3B). Following induction, treatment with 20 µg/mL CTHRC1 significantly increased calcified nodule formation compared to the control group and the group treated with 5 µg/mL CTHRC1 (p < 0.05).
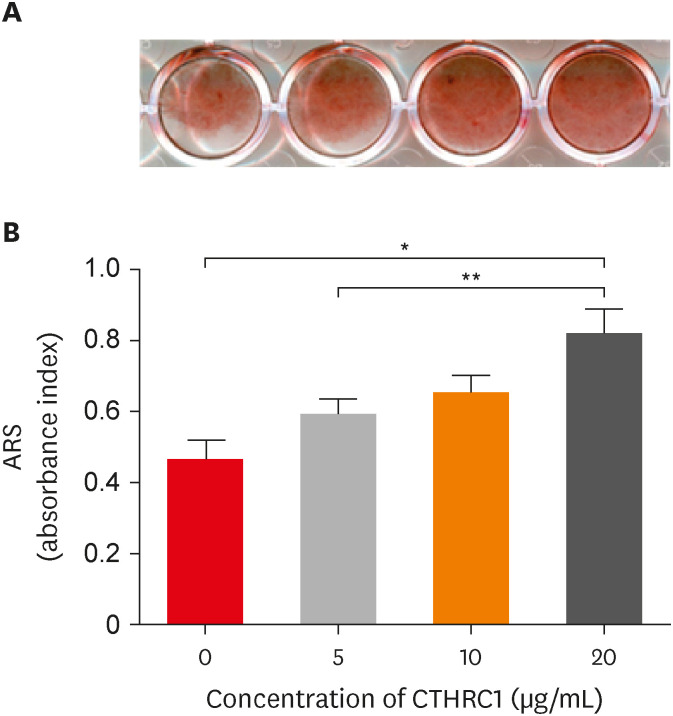
Results of Alizarin red S staining (21 days). (A) Cells were treated with or without CTHRC1 (5, 10, or 20 µg/mL) in OM medium (50 mg/mL ascorbic acid, 10 mmol/L β-glycerophosphate). (B) The formation of mineralized nodules and calcium deposits significantly increased at 5 and 20 µg/mL concentrations of CTHRC1 (p < 0.05).
CTHRC1, collagen triple helix repeat containing 1.
CTHRC1 promoted cell migration of hDPSCs
hDPSCs were treated with CTHRC1 at 10 and 20 µg/mL for 24 hours. As depicted in Figure 4, the wound healing assay showed that CTHRC1 treatment led to significantly greater healing over the scratch than was observed in the control group (p < 0.05). The extent of wound closure also showed a significant increase in both the control and CTHRC1 groups from 0 to 24 hours (p < 0.05).
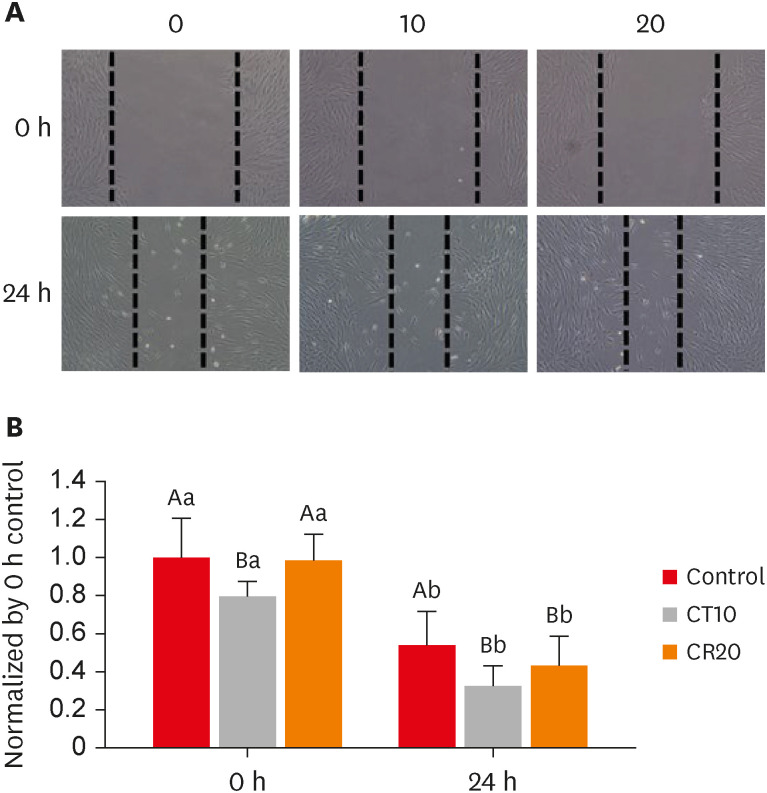
CTHRC1 promotes cell migration of hDPSCs. (A) Scratch wound healing assays were performed in hDPSCs treated with CTHRC1. Representative microscopic views at 0 and 24 hours are shown. (B) The quantitative analysis of the decreased gap area after 24 hours shows a significant difference. Different capital letters denote significant differences among treatment groups at the same time. Different small letters denote significant differences in each group over time from 0 to 24 hours (p < 0.05).
CTHRC1, collagen triple helix repeat containing 1; hDPSCs, human dental pulp stem cells.
DISCUSSION
The pulp healing process involves complex molecular and cellular interactions. After a severe injury, dental pulp stem cells must differentiate into secondary or replacement odontoblasts, followed by dentinogenesis, for the dentin-pulp complex to regenerate. Dental pulp stem cells have the ability to renew themselves and differentiate into multiple lineages [23]. A complex network of signaling molecules, pathways, and receptors regulates this process of cellular differentiation [24].
Several studies have shown that CTHRC1 is associated with numerous physiological and pathological processes, including the formation of new bone, morphogenesis during development, inflammatory arthritis, and the progression of cancer [252627]. One study showed that CTHRC1 transgenic mice exhibited a higher bone mass increase due to elevated osteoblast bone formation, while CTHRC1-deficient mice showed lower bone mass [21]. However, little is known regarding the effects of CTHRC1 on the odontogenic differentiation of hDPSCs.
The aim of this study was to provide evidence supporting CTHRC1 as a potential candidate for effective dental pulp complex regeneration therapy. Therefore, we first examined whether CTHRC1 could enhance cell mobility without impacting cell proliferation. We also performed wound healing assays to validate that recombinant CTHRC1 increases the speed of healing. The cell migration assay confirmed that CTHRC1 enhanced cell mobility relative to the control group. Furthermore, the data on proliferation demonstrated that recombinant CTHRC1 did not significantly affect the proliferation of hDPSCs up to a concentration of 10 µg/mL. Next, in the wound healing model, 10 µg/mL CTHRC1 achieved satisfactory wound healing. These results provide evidence that recombinant CTHRC1 is capable of promoting wound healing.
Angiogenesis is the most crucial step during the healing process, as it ensures the delivery of oxygen and nutrients to the injured area. The expression of various vascular growth factors and modulators, including VEGF and FGF-2, regulates angiogenesis [28]. In the present study, CTHRC1 did not affect the expression of VEFG and FGF-2 in hDPSCs. It seemed that CTHRC1 exerted a chemotactic effect on hDPSCs, but no effect on angiogenesis.
The mRNA expression levels of the odontogenic markers DSPP and DMP-1 were quantified as a measure of odontogenic differentiation in hDPSCs after CTHRC1 treatment [29]. During the formation of dentin, DSPP and DMP-1 are involved in the nucleation and formation of hydroxyapatite [30]. The mRNA expression of these markers was upregulated. DSPP and DMP-1 belong to the small integrin-binding ligand N-linked glycoprotein family. DMP-1, which is expressed early during the process of osteoblastic differentiation, plays a role in the matrix mineralization of bone and dentin [31]. Generally, tooth formation is achieved through angiogenic-odontogenic coupling [32]. The results of this study are not consistent with previous studies. Studies have shown that CTHRC1, as a secreted protein, promotes tumor invasion and metastasis via tumor angiogenesis [33]. The effects of CTHRC1 are exerted through several signaling pathways, including transforming growth factor-β, Wnt, integrin β/focal adhesion kinase (FAK), Src/FAK, mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (ERK), phosphoinositide 3-kinase/AKT/ERK, hypoxia-inducible factor-1α, and protein kinase C-δ/ERK [33].
In summary, extracellular CTHRC1 did not affect angiogenesis, but might have accelerated odontogenic differentiation in hDPSCs. This may be due to differences in cell types. hDPSCs have a higher propensity for odontoblastic differentiation than angiogenesis, compared to tumor cells. Further in vivo and in vitro studies are necessary to identify the signaling pathway that caused those outcomes. The experimental results show that angiogenesis and the odontogenic differentiation of hDPSCs, which are required for pulp regeneration, were not properly achieved. Thus, the CTHRC1 used in this study seems unsuitable as a material for pulp regeneration.
CONCLUSIONS
The data indicate that CTHRC1 had no significant effect on the proliferation of hDPSCs, except at the highest dose (20 µg/mL). Calcific nodule formation was increased by 20 µg/mL CTHRC1. Further, a 10 µg/mL concentration of CTHRC1 promoted cell migration of hDPSCs.
Notes
Funding: This research was funded by a Chonnam National University (GN: 2020-1833) and National Research Foundation of Korea grant funded by the Korean government (NRF-2020R1F1A1048271).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Hwang YC.
Data curation: Kim JS.
Formal analysis: Lee BN.
Funding acquisition: Hwang YC.
Investigation: Kim JS.
Methodology: Kim JS.
Project administration: Oh WM.
Resources: Hwang IN.
Software: Chang HS.
Supervision: Hwang YC.
Validation: Lee BN.
Visualization: Chang HS.
Writing - original draft: Kim JS.
Writing - review & editing: Hwang YC.


