Correlation between different methodologies used to evaluate the marginal adaptation of proximal dentin gingival margins elevated using a glass hybrid
Article information
Abstract
Objectives
This study aimed to evaluate the effect of aging on the marginal quality of glass hybrid (GH) material used to elevate dentin gingival margins, and to analyze the consistency of the results obtained by 3 in vitro methods.
Materials and Methods
Ten teeth received compound class II cavities with subgingival margins. The dentin gingival margins were elevated with GH, followed by resin composite. The GH/gingival dentin interfaces were examined through digital microscopy, scanning electron microscopy (SEM) using resin replicas, and according to the World Dental Federation (FDI) criteria. After initial evaluations, all teeth were subjected to 10,000 thermal cycles, followed by repeating the same marginal evaluations and energy dispersive spectroscopy (EDS) analysis for the interfacial zone of 2 specimens. Marginal quality was expressed as the percentage of continuous margin at ×200 for microscopic techniques and as the frequency of each score for FDI ranking. Data were analyzed using the paired sample t-test, Wilcoxon signed-rank test, and Pearson and Spearmen correlation coefficients.
Results
None of the testing techniques proved the significance of the aging factor. Moderate and strong significant correlations were found between the testing techniques. The EDS results suggested the presence of an ion-exchange layer along the GH/gingival dentin interface of aged specimens.
Conclusions
The marginal quality of the GH/dentin gingival interface defied aging by thermocycling. The replica SEM and FDI ranking results had stronger correlations with each other than either showed with the digital microscopy results.
INTRODUCTION
In response to the increasing demand for dental esthetic solutions, the development of tooth-colored materials and dentin adhesives has progressively advanced [1]. Unfortunately, this has created new problems, including proximal cavities with dentin-gingival margins, which are common clinical problems [2]. Subgingival cavities with cervical margins extending below the cemento-enamel junction (CEJ) generate significant technical and operative challenges, including the loss of partial or total sealing of the cervical margins in the absence of enamel [3]. Adhesive bonding to the etched enamel is efficient and stable; however, adhesion to dentin is more challenging due to the high organic component, tubular structure, permeability, and low surface energy of dentin [45]. Consequently, bonding to deep cervical dentin and keeping the margins of any adhesive restoration sealed over time cannot be considered entirely predictable and safe [6].
The elevation of proximal dentin margins under either direct or Indirect restorations has been investigated using either glass ionomer (GI)-based or resin-based materials [78]. Several studies have advocated the use of resin composites for bonding with such margins, especially with existing xlink:types of adhesives [2]. Resin composites have shown the best mechanical properties in terms of elastic modulus, flexural strength, hardness, and wear resistance [9]. However, some recent studies have argued that GIs, with the hydrophilic nature, flexibility, chemical bonding, and enhanced mechanical and wear properties of their newer generations, could be a more suitable option for bonding to deep moist dentin/cementum margins [1011]. One of the promising newer-generation GIs is a glass hybrid (GH) (EQUIA Forte Fil; GC Corp., Tokyo, Japan). This product is reinforced by the presence of evenly dispersed ultrafine and highly reactive glass particles and optimization of the molecular weight of the polyacrylic acid, leading to excellent mechanical properties that enable its safe use in high-load-bearing areas [12]. Indeed, Moshaverinia et al. [13] reported that EQUIA Forte Fil is a promising restorative material with superior flexural strength and surface hardness compared with its predecessor, Fuji IX GP (GC America Inc., Alsip, IL, USA) or other commercially available GIs.
The marginal integrity of restorative materials is an important parameter when predicting long-term behavior [14]. The evaluation of an in vitro marginal seal can be performed by either measuring microleakage or evaluating the marginal adaptation [15]. Dye penetration testing lacks clinical relevance and inter-study correlations compared to microscopic marginal analysis [15]. Scanning electron microscopy (SEM) has been the backbone of the research conducted on the resin-dentin interface; however, it is an invasive technique, in addition to the prior vacuum processing that may cause dryness and cracking of the specimens [16]. Several studies have tried to circumvent these limitations by using the replica technique, where epoxy die replicas for the specimens were examined instead of the specimens themselves [615]. This would enable retesting of the same specimens at different time points, allowing for monitoring restorations over a longer time span [15]. However, Al-Harbi et al. [15] showed disadvantages of the replica technique, especially related to the accuracy, as the manipulation of both impression and replication materials may mask or alter some structures and hence affect the details at higher magnification. Furthermore, the correlation between SEM marginal analysis and clinical bonding was found to be only low or moderate [17].
Recently, digital scanning microscopy has been used for the marginal analysis of restoration/dentin interfaces [10]. It is a non-destructive, simple, and quick method and can be used directly on the specimens without replicas [10]. Moreover, it does not need a strictly flat surface when used at higher magnifications. In order to provide more clinically relevant testing for marginal integrity, Heintze [18] suggested using loupes and explorers for the assessment of the marginal integrity of restorations and rating them according to the World Dental Federation (FDI) ranking criteria. This has the advantage of allowing testing at different stages of a study, in addition to saving time and cost [18]. Although there are some data in the literature discussing the association between clinical and SEM marginal analyses, the correlation between marginal analysis using digital microscopy and other clinical or microscopic methods of marginal evaluation for direct restorations has not been reported previously in the literature [18].
Based on the previous data, a question might be raised: which of the marginal testing methods provides more useful and clinically relevant evidence, and even more interestingly, do different in vitro methods performed on the same specimens give consistent results? In addition, recent xlink:types of GI restorations seem to have many characteristics that would be beneficial in the subgingival environment [10]. However, there is little information in the literature regarding their use in deep margin elevation. Therefore, the aim of this study was to evaluate and compare the marginal quality of proximal dentin gingival margins elevated using a recently developed GH, and to analyze the consistency of the results obtained by 3 in vitro methods commonly used for such evaluations. The null hypotheses were: 1) the aging condition would not affect the marginal quality when the GH is bonded to proximal dentin gingival margin; and 2) there would be no correlation between the results of the marginal quality obtained from the 3 different testing methodologies for the same specimens either before or after aging.
MATERIALS AND METHODS
Materials
One commercially available GH restorative material was tested in the current study (EQUIA Forte HT Fil; GC Corp.). Additionally, 1 universal adhesive (Adhese Universal; Ivoclar Vivadent, Amherst, NY, USA) and 1 nanohybrid bulk fill resin composite material (Tetric PowerFill; Ivoclar Vivadent) were used. A detailed description of the materials is presented in Table 1.
Cavity preparation
Ten human upper molars recently extracted due to periodontal disease were included in this study; they had approximately similar dimensions and were examined under a stereomicroscope to confirm they were caries- and crack-free. They were cleaned with an ultrasonic scaler to remove soft tissue and calculus deposits, then stored in 0.5% chloramine T solution until used. All samples were used within 1 month of their extraction. The teeth were collected after receiving approval from the Institutional Review Board of the University of Tennessee, Health Science Center (No. 21-08400).
The roots of all teeth were fixed vertically in fast self-curing acrylic blocks (SamplKwick; Buehler, Lake Bluff, IL, USA) 2 mm below the CEJ, to facilitate the preparation and restoration steps. Compound class II cavities with standardized dimensions were prepared on the mesial surfaces of all teeth. This was done using a cylindrical medium grit diamond bur (2284879; Kerr Corp, Orange, CA, USA) and finished with a 25 µm finishing diamond under copious water coolant with a high-speed handpiece (SN 2018-1041978; W&H, Bürmoos, Austria). A pencil was used to mark the outline before preparation. The cavity dimensions were as follows: occlusal: 3-mm bucco-lingual width, 3-mm depth; box: 1 mm below the CEJ, 1.5-mm mesio-distal dimension at the bottom, 3-mm bucco-lingual width. The margins were not beveled but had slightly rounded line angles (Figure 1A). New burs were used after the preparation of every 5 cavities. The dimensions were calculated using a graduated periodontal probe (PCPUNC127; Hu Friedy, Chicago, IL, USA) [4]. After preparation, the cavities were examined for any defects. The buccal and palatal walls of the proximal boxes of all teeth were marked with a pencil 1 mm above CEJ (to mark the level of the base material) (Figure 1B).
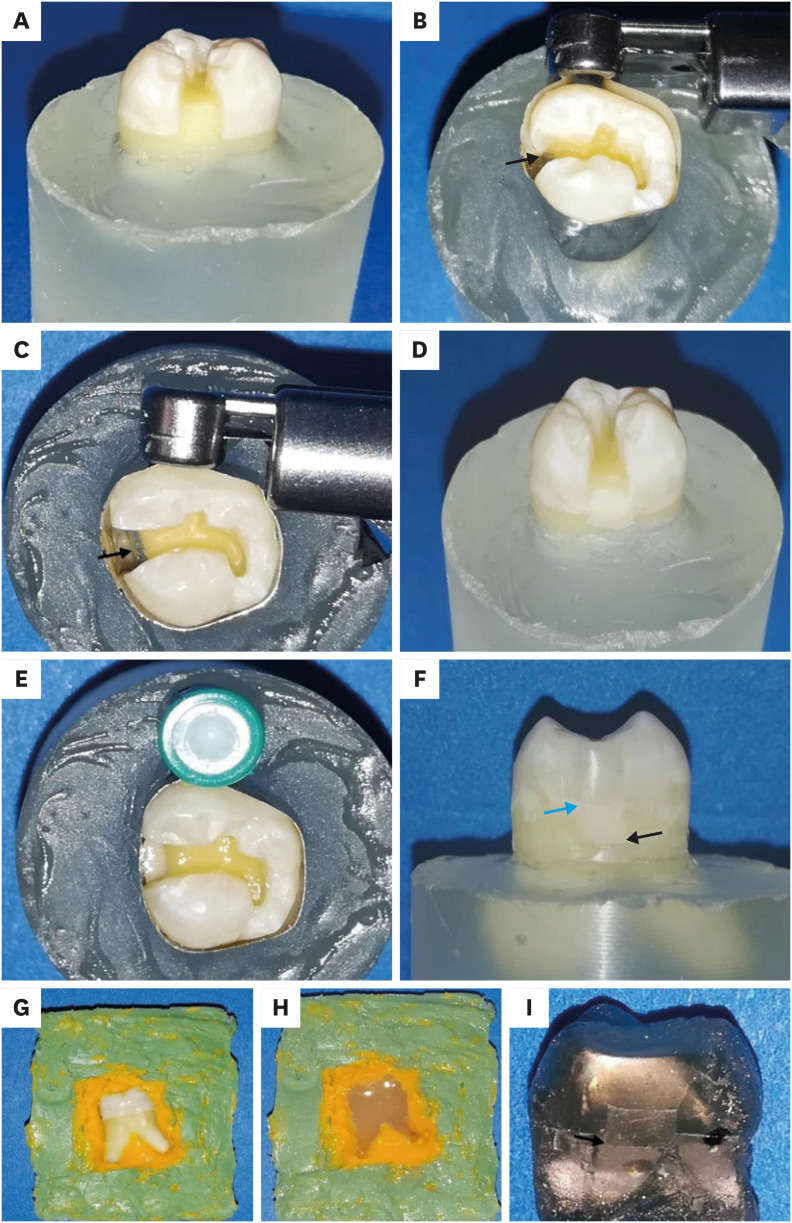
Methodology for a representative specimen. (A) Proximal cavity outline and dimensions, cervical margin 1 mm below the cemento-enamel junction (CEJ). (B) Occlusal view of the Tofflemire matrix band (black arrow: the pencil mark 1 mm above the CEJ). (C) Occlusal view of the Tofflemire matrix band (black arrow: intimate adaptation against the gingival margin). (D) Proximal view after glass hybrid (GH) placement. (E) Occlusal view after a new circumferential matrix system and universal adhesive application. (F) Proximal view of the final restoration after overlying resin composite placement and finishing and polishing (black arrow: the GH/gingival margin interface and blue arrow points to GH/overlying resin composite interface). (G) Recording of the GH/gingival dentin interface using addition silicone impression materials. (H) Pouring the impression with epoxy resin for creating the resin replica. (I) Proximal view after gold sputter coating for the resin replica (black arrows: the GH/gingival margin interface).
Restorative procedures
After the preparation procedures, the cavities were washed with water and dried. The gingival, buccal, palatal, and axial dentin margins of the proximal parts of the cavities were conditioned as recommended by the manufacturer with a dentin conditioner (GC Corp.) for 10 seconds. This was followed by rinsing and drying. The occlusal and proximal enamel margins of all cavities were selectively etched with 37% phosphoric acid (N-Etch, Ivoclar Vivadent) for 15 seconds, rinsed with water for the same time, and then gently dried in oil-free air without desiccation.
The restorations were performed in 2 steps: the first for GH base material application and the second for restoring the remaining cavity. A Tofflemire matrix band (Fintrec Dead Soft Matrix; Pulpdent Corp., Watertown, MA, USA) was contoured and placed around each tooth while making sure that the end of the band was beyond the gingival margin of the cavity. The ultrathin soft metallic bands were burnished against the gingival margin to provide the maximum adaptation, which was checked visually with magnification (×4) and in a tactile manner using the tip of a dental explorer (Shepherd Hook; Premium Instruments, Ronkonkoma, NY, USA). This was done to prevent the creation of gross marginal discrepancies (Figure 1C). Next, all teeth were restored up to 1 mm above the CEJ with the GH material in a bulk technique. A titanium-coated condenser (1.5 mm; Artman Instruments, Kennesaw, GA, USA) was used to gently tap on the material’s surfaces during restoration to ensure adaptability against gingival margins (Figure 1D). The base material was mixed and dispensed according to the manufacturers’ instructions. The Tofflemire matrix-band was changed to another circumferential matrix system (No. 2162, HAWE SuperMat, Kerr Corp.). Next, the universal adhesive was applied after GH placement on the rest of the cavity surfaces and on the GH surface that would be bonded to the overlying resin composite and air-thinned and light-cured as recommended by the manufacturers’ instructions (Figure 1E). The curing procedure was performed using an LED curing light (Elipar Deep Cure; 3M ESPE, St. Paul, MN, USA) operating at 1,000 mW/cm2, checked periodically after every 5 samples with a radiometer (Demetron L.E.D. Radiometer, Kerr Corp.). The remaining cavity was restored with the nanohybrid bulk fill resin composite. It was inserted in the cavity in 2 horizontal increments using a composite placement instrument (TIN206; Brasseler, Savannah, GA, USA) until the cavity was filled [19]. Each increment was cured from the occlusal surface for 20 seconds. Additional curing for 40 seconds was performed from the proximal surface after the removal of the matrix band.
All specimens were stored in distilled water at 37°C for 24 hours in an incubator (Isotem; Thermo Fisher Scientific, Waltham, MA, USA) prior to the finishing and polishing procedures [20]. Finishing and polishing of the restorations and removal of any visible overhangs were performed with Al2O3 discs (Extra-Thin Sof-Lex discs, 3M ESPE) using a low-speed handpiece (A4209792; Brasseler USA, Tochigi-ken, Japan) under water cooling (Figure 1F). All specimens were then kept in an incubator for 1 week at 37ºC. All specimens were removed from their fixation blocks and cleaned ultrasonically before further testing. All preparation and restoration procedures were performed by a single operator using magnification (×4 loupes; Amtech, Wenzhou, China) and LED headlight illumination. Teeth were randomly numbered from 1 to 10 on both the buccal and palatal surfaces of the crown to facilitate the comparison of pre- and post-aged gingival margins of the same tooth.
Marginal adaptation evaluation using a digital microscope
All specimens’ GH/dentin gingival interfaces were examined under a digital microscope (VHX-1000 and VH-Z20R lens; Keyence Corporation, Osaka, Japan). The specimens were fixed on the stage using a piece of sticky wax (Kerr Corp.), and the motorized stage was then moved until the required interface appeared on the screen. This was followed by adjusting the focus manually until the interface appeared with no shadow effect. If a shadow appeared at the interface, the lens was tilted 30° until the shadow disappeared and the interface became clear [21].
An overall proximal view of the margins between the GH and tooth structure was captured at ×30. Then, a fully focused HDR image for each part of the interface was captured and measured at ×200 after setting the upper and lower limits of focus. The vertical pitch was adjusted automatically. Finally, the sectioned images were stitched together using the 3-dimensional image stitching option. This was done to obtain a single image of all parts of the interface for each tooth with the same magnification (×200) [21]. The contrast of all stitched images was adjusted to 50%. The marginal quality of each GH/gingival dentin interface was expressed as the percentage of continuous margin (% CM) (length of the perfect margin, in millimeters)/[length of the perfect margin + length of the imperfect margin] × 100). The marginal quality was classified as continuous or gap-free (exhibiting a gap of less than 1 µm) or discontinuous or containing a gap (exhibiting a gap more than 1 µm wide). This was conducted according to a well-proven protocol for describing margins in gingival dentin [1622]. Areas that could not be judged were excluded. In addition, any cohesive failures that occurred within the GH near the gingival margin were recorded as gaps. Before measurements were made, the digital microscope and the examiner were calibrated by repeated trials. All measurements were performed using the device’s software (VHX-H1M1, Keyence Corporation).
Marginal adaptation evaluation using SEM
The mesial restored surfaces of all teeth were cleaned with ethanol and dried. Then, these surfaces were recorded using addition silicone impression materials, of heavy and extra-light fast set consistencies (Aquasil Ultra+; Dentsply Sirona, Charlotte, NC, USA) with a 2-step double mix technique (Figure 1G). The impressions were left to polymerize, then removed from the specimens following the manufacturers’ recommendations. The impressions were then allowed to fully polymerize over 12 hours [11]. Next, each impression was poured with epoxy resin (Hardman Double/Bubble extra-fast set; Ellsworth Adhesives, Germantown, WI, USA) using a vortex (VWR, Atlanta, GA, USA) to eliminate voids (Figure 1H). All replicas were left to dry at room temperature for 24 hours, after which they were removed from the impressions, trimmed, and cleaned with ethanol [15]. This was followed by mounting on aluminum stubs, and sputter coating with gold (Denton Vacuum LLC, Moorestown, NJ, USA) (Figure 1I). The overall proximal view of the margins between the GH and tooth structure was examined at ×20 under SEM (Zeiss EVO HD15; Carl Zeiss, Oberkochen, Germany). Each part of the GH/gingival dentin interface was examined and measured at ×200 magnifications, using the same criteria as in the digital microscope evaluation. Images were analyzed with the device’s image analysis software (SmartSEM v6.05, Carl Zeiss). Both marginal adaptation evaluations and measurements using the former 2 techniques were performed by 1 operator who was experienced with quantitative margin analysis and who was not informed of the restorative procedures. The same measurement procedures for both techniques were repeated by the same examiner after 2 weeks to assess the intra-examiner reliability of the measurements (intraclass correlation coefficient; ICC).
Marginal adaptation evaluation using FDI criteria
The quality of all GH/gingival dentin interfaces was assessed with the aid of magnifying loupes (×4), powerful light sources, and 2 special dental explorers with different blunt tips (150 and 250 μm) (150EX and 250EX; Deppeler SA, Rolle, Switzerland). The marginal quality was ranked according to the FDI criteria (Table 2) [23]. All specimens were evaluated by 2 examiners who were not informed of the restorative procedures. Training on marginal ranking using the FDI criteria was performed during the pilot study. Inter-examiner and intra-examiner agreement of at least 90% was required before the beginning of the evaluation [24]. After the evaluation, inter-examiner reliability was assessed with Cohen’s kappa coefficient.
Artificial aging
After GH/dentin gingival interfaces assessment, all teeth were artificially aged by thermocycling for a total number of 10,000 cycles (Sabri Dental Enterprises Inc., Downers Grove, IL, USA), which represents approximately 12 months of clinical service [25]. The specimens were alternated between 5°C and 55°C ± 2°C according to ISO 11405 (International Standards Organization) recommendations, with the water temperature continuously checked [26]. The dwell time was 20 seconds in each bath and the transfer time was 10 seconds between baths [26]. Finally, all specimens were carefully evaluated under an optical microscope to check for cracks.
Marginal adaptation evaluation after aging
All marginal adaptation evaluations of the GH/gingival dentin interfaces of all specimens were repeated using the 3 techniques. This was done with the same parameters and criteria used in the pre-aging evaluations.
Morphological and chemical analysis of interfacial zone at the GH/gingival dentin interface after aging
Although the primary aim of the current study was the quantitative marginal analysis of the GH/gingival dentin interface, a distinct layer was repeatedly noticed along the interfacial zone in the aged specimens. It had clearly different morphology than either GH or gingival dentin and ranged in size from 1.5–2.5 μm. It was observed in both digital and SEM evaluations but was more obvious in the SEM images. In order to conduct a morphological and chemical microanalysis of this layer, SEM-energy dispersive spectroscopy (EDS) was used. Two aged specimens were selected, as they had the most distinct layers at the interface on the microscopic evaluations. This was followed by sputter coating using the same steps as previously mentioned. SEM examinations were then done at ×3,000, along with a microanalysis of 9 different spectra in each specimen, 3 along the GH, 3 along the interfacial zone, and 3 along the gingival dentin part. This was conducted using a SEM-EDS system (Oxford Instruments, Abingdon, UK) and software (aZtecLive; Oxford Instruments).
Statistical analysis
1. Sample size calculation
The sample size for this study was calculated initially before conducting any work using the G*Power program (Ver. 3.0.10; G*Power, Kiel, Germany) based on a previous study with a similar design [15]. The total sample size of 10 teeth achieved 80% power (equal to xlink:type II error), and the xlink:type I error (α) was 0.05.
2. Statistical methods
The data were statistically analyzed using SPSS version 20 (IBM Corp, Armonk, NY, USA). The intra-examiner reliability was tested by the ICC, which was calculated for measurements of the % CM data from digital microscopy and SEM evaluations. The Cohen kappa statistic was used to measure the agreement between the 2 examiners in the FDI ranking evaluation. The % CM values for both microscopic techniques proved to be normally distributed after they were subjected to the Shapiro–Wilk test and the homogeneity of variances was tested using the Levene test. Thus, parametric tests were used to compare % CM values for both microscopic techniques of the study groups (pre- and post-aging), and non-parametric tests were used to compare the groups’ FDI ranking. The effect of aging on % CM values in each technique was evaluated using the paired-sample t-test (at p < 0.05). Meanwhile, the Wilcoxon signed-rank test was utilized to compare the FDI ranking of the 2 groups, with a significance level of 5%. Finally, Pearson correlation coefficients (ρ) were used to evaluate the correlations of the digital microscopy and SEM results, while Spearman correlation coefficients (ρ) were used to analyze the correlations of each microscopic technique’s values with the FDI ranking results.
RESULTS
The ICC for the 2 measurements of % CM data was 0.994 and 0.997 for digital microscopy and SEM, respectively, representing excellent reliability; therefore, the average of both sets of measurements for each technique was used for further analysis. Regarding the agreement between the 2 examiners for the FDI ranking, the overall Cohen kappa statistics revealed satisfactory agreement between the 2 examiners for the immediate group (0.90) and the aged group (0.94).
Results of % CM using digital microscopy and SEM
The mean % CM values and standard deviations for the study groups using microscopic techniques are presented in Table 3. The paired-sample t-test revealed that there was no significant difference between the study groups according to both microscopic evaluations (p > 0.05); however, the aged group had lower % CM values than the immediate group. The results of the t-test for both groups using microscopic techniques are presented in Table 4. The Pearson correlation coefficient between the % CM values of the microscopic techniques showed a significant, moderate positive relationship (ρ = 0.469, p < 0.05). Representative digital microscopy and SEM images for GH/gingival dentin interfaces of both groups are presented in Figures 2 and 3. In the immediate group, detached particles from the material at the GH/gingival dentin interface were repeatedly observed (Figure 2, B6). In addition, many of the gaps at the margins were within the material itself, as if they were caused by cohesive failure (Figure 2, B7, and B8).
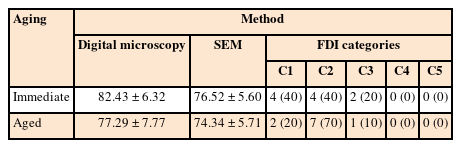
World Dental Federation (FDI) categories of marginal adaptation evaluation results using the 3 techniques immediately and after aging
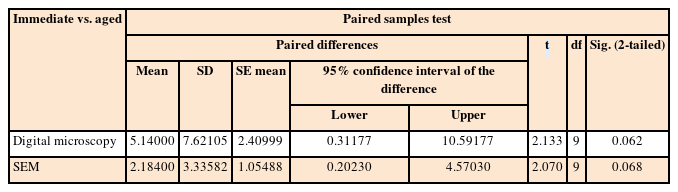
Comparison of percentage of continuous margin values between the immediate and aged groups evaluated using digital microscopy and scanning electron microscopy (SEM)
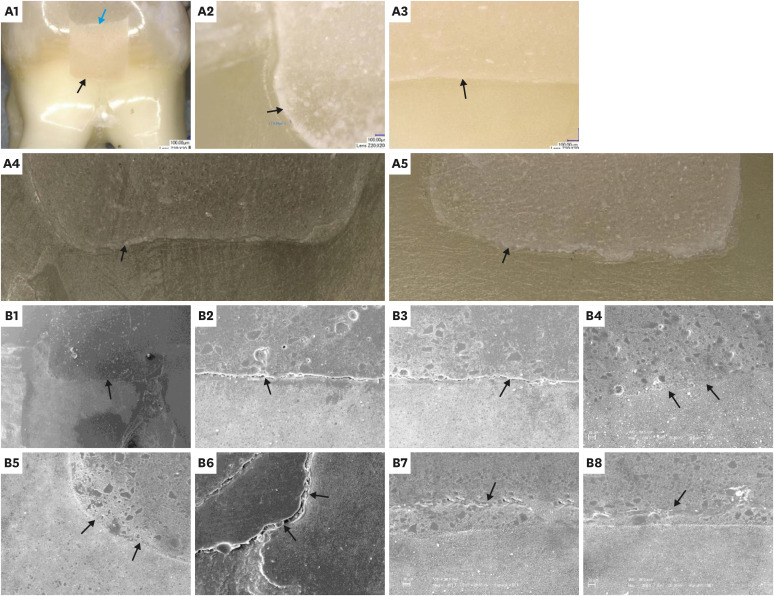
Representative digital microscopy (A) and scanning electron microscopy (SEM) (B) images of the immediate group: Overall views (A1, B1) at the glass hybrid (GH)/gingival dentin interfaces (A2-5, B2-8). A1: Digital microscopy, overall view at ×30 (black arrow: the GH/gingival dentin interface; blue arrow: the GH/overlying resin composite interface). A2: Example of a gapped GH/gingival dentin interface at ×200 (black arrow: marginal gap). A3: Example of a continuous GH/gingival dentin interface at ×200 (black arrow: continuous margin). A4 and A5: Examples of stitched images for the entire GH/gingival dentin interfaces at ×200 (black arrows: GH/gingival dentin interfaces). B1: SEM overall view at ×20 (black arrow: the GH/gingival dentin interface). B2 and B3: Examples of gapped GH/gingival dentin interfaces at ×200 (black arrows: marginal gaps). B4 and B5: Examples of continuous GH/gingival dentin interfaces at ×200 (black arrows: continuous margins). B6: GH/gingival dentin interface at ×200 (black arrows: detached particles from the material at the interface). B7 and B8: GH/gingival dentin interfaces at ×200 (black arrows: gaps within the material, near the margin, representing an example of cohesive failure in the material itself).
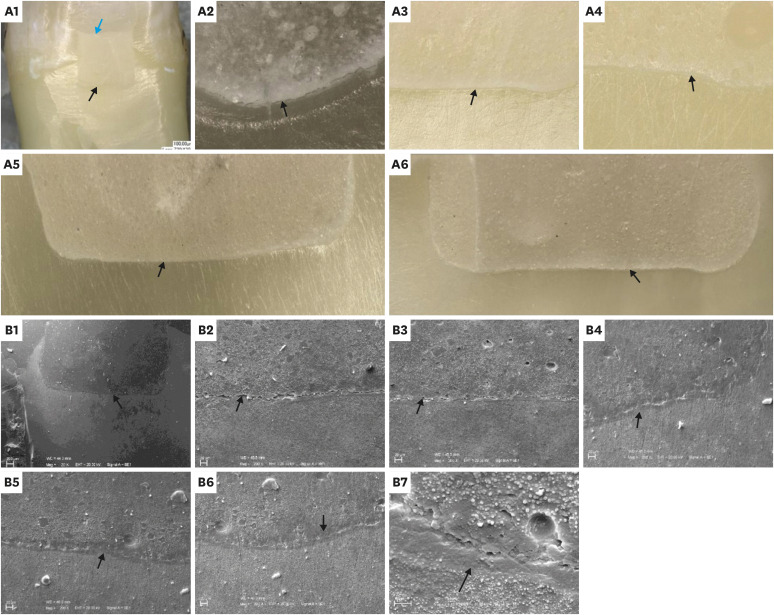
Representative digital microscopy (A) and scanning electron microscopy (SEM) (B) images of the aged group: Overall views (A1, B1), at glass hybrid (GH)/gingival dentin interfaces (A2-6, B2-7). A1: Digital microscopy, overall view at ×30 (black arrow: the GH/gingival dentin interface; blue arrow: the GH/overlying composite interface). A2: Example of gapped GH/gingival dentin interfaces at ×200 (black arrow: marginal gap). A3: Example of continuous GH/gingival dentin interface at ×200 (black arrow: continuous margin). A4: Example of the ion-exchange zone at the GH/gingival dentin interface at ×200 (black arrow: the zone at the interface). A5 and A6: Examples of stitched images for the whole GH/gingival dentin interfaces at ×200 (black arrows: GH/gingival dentin interfaces). B1: SEM overall view at ×20 (black arrow: the GH/gingival dentin interface). B2 and B3: Examples of gapped GH/gingival dentin interfaces at ×200 (black arrows: marginal gaps). B4-B6: Examples of continuous GH/gingival dentin interfaces at ×200 (black arrows: continuous margins and the ion-exchange zones at the interface with their distinct morphology). B7: GH/gingival dentin interfaces at ×1,000 (black arrow: the ion-exchange zone).
Results of SEM-EDS
Among the EDS data, the elemental distribution of calcium in the interfacial zone and gingival dentin spectra was analyzed, in addition to the strontium percentage in the interfacial zone and GH spectra. The mean percentage of calcium along the 6 spectra of the interfacial zone was 15.73 wt%, while it was 24.46 wt% in the gingival dentin spectra. The mean strontium percentage at the interfacial zone was 1.31 wt% compared to 4.42 wt% in the GH spectra. Overall, the EDS analysis for the interfacial zone showed high peaks of calcium and traces of strontium. SEM images at ×3000 for the interfacial zone, along with graphical representations of the wt% of elements detected at the 9 spectra for each sample, are presented in Figure 4.
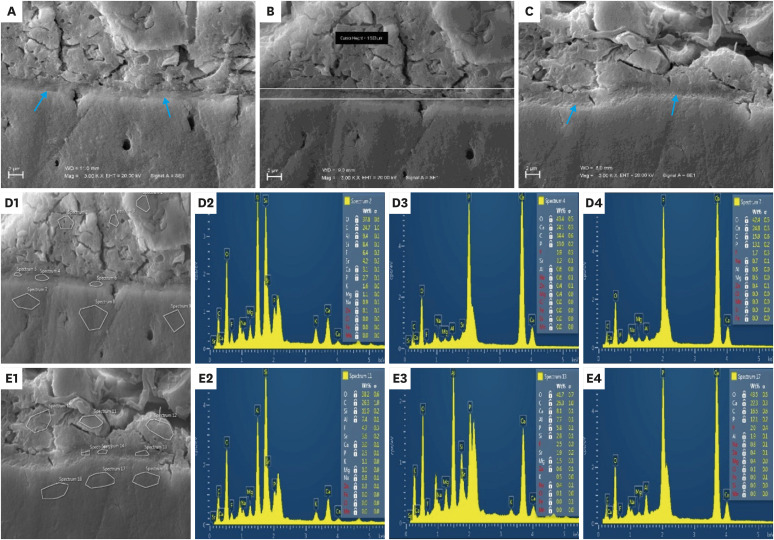
A, B, and C: Scanning electron microscopy (SEM) images for the ion-exchange layer at the glass hybrid (GH)/gingival dentin interface at ×3,000 magnification (blue arrows: the ion-exchange zone). D and E: SEM- energy dispersive spectroscopy (EDS) evaluating the elemental composition of the interfacial zones. D1 and E1: SEM images (×3,000) of the 9 spectra locations for each specimen. D2 and E2: Examples of EDS spectra and tables of elements of GH. D3 and E3: Examples of EDS spectra and tables of elements of the interfacial zone. D4 and E4: Examples of EDS spectra and tables of elements of gingival dentin.
Results of the marginal adaptation evaluation using FDI criteria
The frequencies and percentages for the 5 categories of the FDI ranking of the study groups are presented in Table 3. The Wilcoxon signed-rank test revealed no significant differences between both groups (p > 0.05). Both groups had 100% clinically acceptable scores (1–3). The Spearman correlation coefficients between the FDI ranking and % CM values of digital microscope and SEM showed moderate and strong significant inverse relationships, respectively (ρ = −0.555, p < 0.05 and ρ = −0.813, p < 0.001).
DISCUSSION
In this study, the GH was not placed by filling the whole cavity; instead, it was tested as it would be used clinically. Testing class II restorations with overlying resin composite provide higher C-factors, which lead to greater polymerization contraction stresses; therefore, the specimens were exposed to the challenges of contraction stresses as encountered in clinical situations [4].
A universal adhesive was used to bond the GH to the overlying composite using self-etch (SE) mode. Francois et al. [27] reported that the use of phosphoric acid on the surface of GI can cause weakening of the cement surface and makes it more prone to cohesive failure. In addition, Kandaswamy et al. [28] recommended bonding GI to resin composite using a mild or ultra-mild SE adhesive; they explained that the mild acid attack caused by these xlink:types would result in minimal flushing of ions that can effectively react with the available bonding agent. No resin top coat was added on the surface of the GH specimens to simulate the clinical scenario when elevating the deep proximal subgingival margin, where it could not be applied.
The artificial aging performed in this study was thermal cycling equivalent to 1 year of clinical service [25]. The rationale for choosing thermal cycling was that it could simulate the thermal challenges that these restorations endure due to the mismatch between the coefficient of thermal expansion of each restorative material and the tooth substance [29].
This study focused on the adaptation of GH material against the dentin-gingival margin. The orientation of dentinal tubules, as well as the low hardness and mineralization of proximal gingival dentin, makes this margin the most challenging for proper hybridization and bonding compared to the buccal and palatal dentin margins below the CEJ [430]. In addition, Francois et al. [27] reported an intimate and continuous interface between the same tested GH and resin composite bonded together using a universal adhesive. Microscopic evaluations of marginal adaptation in this study were performed under ×200 magnification, as in previous studies investigating adaptation against the proximal gingival dentin [691522].
Regardless of the marginal evaluation technique, the aging condition did not significantly affect the marginal integrity results. Therefore, the first null hypothesis was accepted. Münevveroğlu et al. [31] reported that water sorption of a non-coated group of highly viscous GI in their study during thermal cycling led to volumetric expansion and marginal sealing comparable to the coated group. This is in agreement with previous studies, which found that non-coated surfaces of GI had higher values of water sorption than coated surfaces, potentially even exceeding the solubility levels of the material [3233]. This might explain the findings of the current study; specifically, the water sorption of GH during thermocycling may have masked some of the marginal defects that could have been caused by aging.
The difference in thermal expansion between the restorative materials and the tooth structure may cause the development of interfacial stresses. This has been implicated as a causative factor in marginal deterioration [34]. Conventional GIs have a linear coefficient of thermal expansion (LCTE) close to that of the tooth structure, which might explain the lack of a significant difference between the current study groups with repeated thermocycling and temperature variations [3435]. Indeed, Pinto-Sinai et al. [34] reported that, based on the LCTE of conventional highly viscous GI, the use of these materials might be preferred for restoring the cervical areas of the molars.
Long-term studies have shown that an ion-exchange process occurs at the interface between the GI cement and the tooth, resulting in the formation of a distinct interfacial region over time [36]. The analysis of this layer when a strontium-based GI cement was used showed that it contained both strontium and calcium, indicating that this zone resulted from the movement of ions from both the cement and the tooth [3738]. Since the calcium could only have come from the tooth surface and the strontium from the cement, the term “ion-exchange layer” was coined to describe this layer [36].
During the current microscopic evaluations of the GH/gingival dentin interfaces, a distinct interfacial zone with characteristic morphology was observed in the aged specimens. Since the GH used in the current study is strontium-based, the fact that the interfacial region contains both calcium and strontium can also be considered evidence for an ion-exchange process. Visual evidence of the morphologies of these zones is that there were immature ion-exchange layers, a finding that arises from the limited aging time. The development of ion-exchange layers is usually caused by the diffusion of respective cations into the interfacial zone and their reaction with appropriate anions to form mechanically strong acid-resistant structures [3839]. The formation of these layers in the aged specimens might have been one of the causes of the preserved marginal quality after thermocycling; indeed, it has been reported that this layer may cause the high durability of the adhesive bond in GIs [36].
Conventional GIs, including the GH tested herein, are highly susceptible to dehydration, especially during the early setting period [31]. Hence, in clinical service, a surface coating is needed to maintain water balance and reduce the resulting dehydration cracks [3140]. However, such a coating was not used in the current study. This means that dehydration could have occurred, which might explain the observation of detached particles of GH at the gingival dentin interface in the immediate group. Furthermore, there were many gaps within the material itself, not exactly at the interface. Using a conditioner prior to applying the GH creates strong bonds to dentin, and these seem mainly to fail cohesively when failure mode was tested [41]. Hoshika et al. [42] confirmed this finding when using the current GH.
Regardless of the group tested, there were significant moderate and strong correlations between the testing techniques’ results for the same specimens. Consequently, the second null hypothesis was rejected. An actual clinical evaluation of the marginal quality of subgingival restorative margins is challenging and most of the time could not be conducted, either directly or with the impression and replica technique [43]. This is especially true after normal gingival healing in cases where there were deep margins [4445]. Therefore, the results of marginal integrity evaluation using explorer and magnifying loupes of extracted teeth with restorations below CEJ might be partially correlated to the clinical outcome [18]. Based on the current study results, the SEM % CM results had a strong inverse correlation with the marginal evaluation based on the FDI criteria. However, the digital microscopy % CM results had a moderate inverse correlation with the other techniques. Although the SEM evaluation technique involves a number of unavoidable drawbacks, the results were more strongly correlated with the results of the clinical simulation than with those of digital microscopy [15]. Contrary to the current findings, Juloski et al. [46], concluded that SEM examination did not allow predictions of the functional sealing of cervically relocated margins. The former study correlated SEM results with microleakage using dye penetration scores of the same teeth. Considering the previously reported systematic search proving that microleakage tests with dye penetration were not correlated with any of the clinical parameters, this might explain the difference in the results [1718].
The present in vitro study has limitations; for instance, this study tested only 1 material, but this study was not designed to evaluate different products, but rather the effect of aging on GH tested and the correlations of different methodologies for the marginal analysis of this new material category. Additionally, the aging in the current study involved only thermocycling without mechanical load cycling, which may have efficiently caused artificial aging. In addition, the qualitative elemental analysis of the ion-exchange layer observed at the interface of the aged group was not studied in detail, as it was not one of the main questions aimed to be answered by the current study. However, it is worth further investigation.
The authors of this study recommend investigating the marginal quality of GH with proximal gingival dentin under more challenging aging conditions. There are other recent non-destructive techniques for marginal evaluation, such as micro-computed tomography and swept-source optical coherence. Therefore, it would be valuable to investigate and validate the results when these techniques are applied to elevated proximal dentin gingival margins. Finally, clinical studies are needed concerning the non-invasive evaluation of marginal quality for subgingival dentin/cementum margins elevated by new promising restorative materials.
CONCLUSIONS
It may be concluded that regardless of the marginal evaluation technique, the current tested GH/proximal gingival dentin interfaces were not affected by thermocycling. Furthermore, although the 3 testing techniques for marginal quality revealed the same lack of significance between the study groups’ results, the replica SEM and FDI ranking marginal quality results had stronger correlations with each other than either with the digital microscopy results.
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Erno Lindner, Professor of Biomedical Engineering, Department of Biomedical Engineering, University of Memphis, USA, for the digital microscopy part of this study and Professor John Nicholson, Director of Bluefield Centre for Biomaterials, London, UK, and Senior Research Fellow, Dental Physical Sciences Unit, Institute of Dentistry, Queen Mary University of London, for reviewing and editing the final draft of this manuscript.
Notes
Funding: This research was funded by the Egyptian Ministry of Higher Education of the Arabic Republic of Egypt as a part of a joint-supervision scholarship offered to the first author.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Ismail HS, Garcia-Godoy F.
Data curation: Ismail HS.
Formal analysis: Ismail HS.
Funding acquisition: Ismail HS.
Investigation: Mehesen R, Ali AI.
Methodology: Ismail HS, Morrow BR.
Project administration: Ismail HS.
Resources: Ismail HS, Morrow BR.
Software: Ismail HS, Morrow BR.
Supervision: Mahmoud SH, Garcia-Godoy F.
Validation: Mehesen R, Ali AI.
Visualization: Ismail HS.
Writing - original draft: Ismail HS.
Writing - review & editing: Mahmoud SH, Garcia-Godoy F.






